THERAPEUTIC
INSIGHTS
Combating Keratitis
A look at the fungal etiologies of this condition.
|
|
|
Coordinated by Bobby Christensen, O.D., F.A.A.O. |
By Larry R. Henry, O.D., Edmond, Okla.
Patients who have keratitis typically complain of red eyes, pain, decreased vision and photophobia. Because this is a common condition, we're familiar with these complaints. The prognosis for keratitis is excellent when we correctly diagnose it and initiate the appropriate treatments early enough. However, correct diagnosis can prove difficult.
Dissecting the problem
So how do we accurately and effectively diagnose and treat this inflammatory condition? We can divide most etiologies of keratitis into infectious causes, such as bacterial and viral, or non-infectious, such as dry eye syndrome, contact lens-related hypoxia and connective tissue disease.
When an episode of keratitis appears resistant to treatment, consider less common etiologies including fungal and parasitic, specifically Acanthamoeba.
This month, we'll explore the fungal etiologies of keratitis and available treatment options. We'll continue with coverage of Acanthamoeba keratitis in November, so look for it then.
A current look at etiologies
Although we've thought that the incidence of fungal and Acanthamoeba keratitis was decreasing, it's actually slightly increasing. Several factors, such as increased international travel, an influx of immigrants from areas of endemic disease, poor contact lens hygiene, regional flooding and difficulty in early diagnosis have contributed to this trend.
Early suspicion of these causes based on patients' case histories and recent advances in diagnosis techniques can help us better manage patients who have atypical keratitis.
Fungal keratitis
Fungal keratitis, although relatively rare in temperate areas such as the United States, is a major cause of infectious keratitis in tropical areas of the world. Fungi can't invade an intact corneal epithelium; therefore, keratitis is associated with diseased corneas, trauma (accidental and surgical) and an altered corneal epithelium resulting from pharmaceutical usage or contact lens wear.
Trauma associated with vegetative matter, such as a tree branch, is by far the most common risk factor for keratitis, regardless of patient age. Other risk factors include contact lens wear (because a fungus can grow within the matrix of soft contact lenses) and systemic illness (especially in children or
immuno-
compromised individuals).
More about fungi
Fungi are divided into filamentous (both septated nonpigmented and nonseptated pigmented), dimorphic and yeast classifications.
Filamentous fungi. Commonly known as molds, filamentous fungi produce feathery, aerial colonies above culture media. They're subdivided into septate and nonseptate organisms. The septated fungi are subdivided into pigmented and nonpigmented groups.
- The non-pigmented, septate, filamentous fungi are the most common causes of fungal keratitis, especially the Fusarium and Aspergillus species.
- The nonseptate filamentous fungi are associated with orbital disease but rarely cause corneal infections.
Dimorphic fungi. This class demonstrates filamentous characteristics when grown at 25ºC and yeast characteristics when grown at 37ºC and are unlikely to cause keratitis.
Yeasts. This class produces creamy, pasty colonies on culture media. Candida is the typical cause of this classification and its incidence increases further away from the equator.
The clinical perspective
The clinical features of fungal keratitis vary among the different species, and you may find them hard to distinguish from bacterial species.
Filamentous fungi produce branching infiltrates with occasional satellite lesions. The cor-neal surface typically appears dry, gray-white in color and has a thickened or elevated epithelium border. You may also notice the presence of an endothelial plaque and hypopyon.
Many believe that the hypop-yon associated with fungal infections is infectious in nature, which may warrant a diagnostic anterior chamber paracentesis to help identify the offending organism. The clinical course of fungal keratitis is chronic. The intrinsic virulence of fungi is attributed to their ability to proliferate within corneal tissue, resist host defenses and produce tissue damage by secreting proteases, hemolysins and exotoxins.
The host inflammatory response also contributes to the tissue damage. This junction of the tissue necrosis and the inflammatory response can produce an immune ring infiltrate.
Fungal keratitis caused by yeasts such as Candida is usually more localized, often with a small ulceration and an expanding infiltrate. It's typically superimposed on a chronic debilitating ocular condition. Candida infections are seen more often in immunocompromised patients.
|
|
|
|
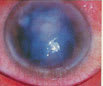
An elevated, pasty, cream-colored infiltrate, as seen in this immunocompromised patient, is characteristic of
Candida. |
|
Identifying the offender
Once you establish a clinical suspicion of fungal keratitis, identify the offending organism from smears and cultures.
Smears. Corneal scraping with a Kimura spatula provides tissue for diagnostic identification, reduces the cornea's fungal load and enhances the penetration of anti-fungal agents. Gram and Giemsa stains are useful for identifying fungi early and KOH preparation and Gomori's meth-enamine silver (GMS) are helpful for detecting fungal elements.
Cultures. Inoculate culture media include sheep blood agar, chocolate agar, Sabouraud's media and thioglycolate broth. Incubate media at both 20ºC and 37ºC for fungal isolation and keep for a period of 3 weeks to rule out any growth. In cases of a negative smear or culture and a high suspicion of fungal keratitis, consider a corneal biopsy.
|
|
|
|
Three weeks after an injury from a tree branch, this slowly healing corneal abrasion |
Attacking the offender
The first successful medical therapy for fungal infections began in 1903 with potassium iodide. The first medications used to treat fungal keratitis weren't developed until the 1950s. Today's anti-fungal medications fall into three classes: polyenes, imidazoles and pyrimidines.
1. Polyenes. This is the first available class of anti-fungal agents. They disrupt the cell wall by preferentially binding to ergosterol in the fungal plasma membrane. Natamycin 5% (Natacyn) and amphotericin B deoxycholate (Fungizone) are the topical agents most often used to fight fungal keratitis.
- Natamycin is the preferred choice against most filamentous fungi, particularly Fusarium and Aspergillus. It's the only anti-fungal commercially available for topical use.
- Add amphotericin B as a second agent if the condition doesn't respond to natamycin alone. It's considered the preferred treatment for Candida infections if there's no clinical improvement with natamycin alone. A 0.15% concentration is compounded from its intrave-nous form for topical use.
Enhance the poor corneal penetration of both natamycin and amphotericin with aggressive corneal scraping. Begin initial treatment with dosing every 30 to 60 minutes for the first 24 to 48 hours. Then, carefully taper the medication according to the clinical response to avoid corneal toxicity from prolonged use. The average course of treatment is 4 to 5 weeks.
2. Imidazoles. These agents inhibit the biosynthesis of ergosterol for cytoplasmic membranes. They're available in oral form but need compounding for topical use. Because of their fungistatic action, only use them as adjunctive therapy.
Oral anti-fungals are recommended for patients who have severe keratitis, scleritis, endophthalmitis or as prophylactic treatment after penetrating keratoplasty for fungal keratitis. If using oral imidazole, monitor the patient's kidneys carefully.
The most common imidazoles used to treat fungal keratitis are miconazole 1% (Monistat), ketoconazole 5% (Nizoral), fluconazole 0.2% (Diflucan) and itraconazole 1% (Sporanox). Consider ketoconazole 200 mg to 600 mg per day as adjunctive therapy for filamentous fungal keratitis and fluconazole 400 mg or itraconazole 200 mg per day for Candida keratitis.
Fluconazole has better ocular tissue penetration, but itraconazole remains in tissues long after administration. Regrettably, itraconazole has little effect against Fusarium, some species of which can produce corneal ulcers.
3. Pyrimidines. Flucytosine (Ancobon), a pyrimidine derivative, works synergistically with other anti-fungals in systemic mycoses. It's the only pyrimidine derivative used in medicine. Never use it alone because it rapidly induces resistance in fungi. It is, however, helpful in treating Candida keratitis in a compounded 2% solution.
|
|
|
|
This fungal keratitis was misdiagnosed as bacterial until corneal cultures identified Aspergillus as the etiology. |
|
The surgical perspective
Roughly 25% of fungal keratitis cases eventually require penetrating keratoplasty because of failed medical therapy or corneal perforation. The goal of surgery is to maintain the structural integrity of the globe and prevent fungal endophthalmitis.
If possible, consider surgery before the infection involves the sclera or limbus. Remove infectious and inflammatory debris from the anterior chamber while injecting amphotericin B or miconazole intravitreally. Post-op, continue the topical anti-fungals, and add oral anti-fungals.
Corticosteroids aren't advisable in treating keratitis because they allow fungi to replicate freely while diminishing the host inflammatory response. They're used to control inflammation and reduce risk of graft rejection, so only use them if you can control the infection clinically. Try cyclosporin A 1% to 4% instead for its anti-inflammatory action and anti-fungal properties.
The visual prognosis for fungal keratitis is bleak when surgical intervention fails. The infection can progress to vitreal involvement and endophthalmitis, and evisceration or enucleation is necessary if treatment of the endophthalmitis fails.
|
|
|
|
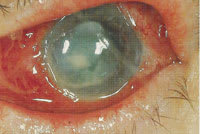
This fungal ulcer, caused by
Fusarium, has a distinct immune ring infiltrate. |
A ray of light
If you identify fungal keratitis early, initiate treatment aggressively to preserve the vision and integrity of your patient's eye. But if medical therapy fails or corneal scarring affects vision, surgical intervention is typically warranted and successful.
Dr. Henry is clinical director for BVA Advanced Surgical Eyecare in Edmond, Okla. He serves as adjunct professor and residency supervisor for Northeastern State University College of Optometry.
Dr. Christensen has a partnership practice in Midwest City, Okla. He's a diplomate in the Cornea and Contact Lens Section of the American Academy of Optometry. He's also a member of National Academies of Practice.