fluoroquinolones
Evaluating the Latest Generation
of Fluoroquinolones
The authors answer the question, "Are fourth-generation fluoroquinolones the 'magic bullet?' "
BY ROBERT DiMARTINO, O.D., M.S., F.A.A.O. &
MEREDITH M. WHITESIDE, O.D., F.A.A.O., Berkeley, Calif.

Successive generations of fluoroquinolones have been produced to counter this problem. How do the newest generation of fluoroquinolones contribute to the management of our patients? Are they indeed the "magic bullet" that we've been looking for and should we now be exclusively using these newest fluoroquinolones?
What is a fluoroquinolone?
A fluoroquinolone is an antibiotic that destroys bacteria by interfering with its DNA replication. Early generation fluoroquinolones hamper bacterial DNA synthesis during replication primarily by inhibiting DNA gyrase, one enzyme required for bacterial (but not human) DNA replication. The current fourth-generation fluoroquinolones markedly inhibit two enzymes (DNA gyrase and topisomerase IV) involved in bacterial DNA replication. The general consensus is that because these agents inhibit both of these enzymes, there's a much lower likelihood that bacterial resistance will develop.

Most ophthalmic literature purports that bacterial resistance to fluoroquinolones, and for that matter antibiotics in general, is directly related to the under-treatment or an incomplete treatment cycle of systemic conditions with oral antibiotics. But it's nonetheless important for us to understand how resistance develops because this knowledge makes it clearer why the use of the newer fluoroquinolones in some cases may be advantageous over continued use of the earlier generations of fluoroquinolones.
Bacteria have developed resistance to the second-generation fluoroquinolones basically by two main mechanisms: The first involves modifying the enzyme targeted by the drug (e.g., DNA gyrase). If a bacterial gene becomes mutated so that it creates an altered but still functional version of DNA gyrase, then an early generation fluoroquinolone that has a strong affinity for only the original form of the enzyme would prove ineffective and allow bacteria with the mutation to proliferate.
The second way resistance has emerged to second-generation fluoroquinolone antibiotics is when the bacteria reduces the fluoroquinolone's access to its target enzyme within the cell. It may achieve this either through the expression of new efflux pumps that remove the fluoroquinolone from the cell or by the cell's membrane acquiring reduced permeability to the fluoroquinolone (Blondau, Hwang). Once again, the result would be for the bacteria with either or both of these new characteristics to proliferate.
In considering acquired resistance to antibiotics or to fluoroquinolones, we should note that a recurrent theme in the systemic and ophthalmic literature emphasizes the importance of achieving high concentrations of the drugs in affected tissues. This provides the greatest chance for the antibiotic to eradicate the infection before mechanisms of bacterial drug resistance can evolve.
The newer fluoroquinolones possess characteristics designed to discourage the development of bacterial resistance. Compared to previous generations of fluoroquinolones, they have an increased ability when topically applied to penetrate and maintain therapeutic levels in ocular tissues, thus improving their efficacy (Hwang). In addition, both gatifloxacin and moxifloxacin bind tightly to two enzymatic target sites -- again making it much less likely that a single mutation could defeat them.
Exercise caution
Second-generation fluoroquinolones exhibited such a rapid kill rate that experts believed that bacteria wouldn't be able to adapt and therefore not become resistant to these agents. Unfortunately, ophthalmic and systemic practice has demonstrated and the current literature supports the conclusion that bacterial resistance to second-generation fluoroquinolones has occurred, most noticeable in Gram-positive bacteria.
This fact leads to the assertion that continued use of second-generation fluoroquinolones would result in an increasing number of bacteria that are resistant to DNA gyrase (the mechanism of action for these antibiotics). If this is true, then bacteria resistant to second-generation fluoroquinolones would only require a single additional mutation to topisomerase IV, the second action of fourth-generation fluoroquinolones, to render these new drugs ineffective.
One long-term strategy in preventing more resistance to second-generation and decreasing the likelihood of resistance to fourth-generation fluoroquinolones might be for clinicians to discontinue the use of the previous generation fluoroquinolones, replacing them to with fourth-generation agents. At the outset, it appears that these new fluoroquinolones may have a significant role in treating bacterial infection and from the standpoint of antibiotic resistance, they may be the most appropriate drug choice.
Examining efficacy
Each generation of fluoroquinolone has different properties. The early fluoroquinolones were effective against Gram-negative bacteria (e.g., Pseudomonas aeruginosa). These early generation agents were less effective against Gram-positive bacteria (e.g., Staphylococcus aureus).
A limited number of reports suggest that second-generation fluoroquinolones have lost their effectiveness to some Gram-negative bacteria. While this remains in question, it's generally agreed that the early fluoroquinolone agents have lost significant ground in their effectiveness against Gram-positive bacteria, all as a result of bacterial resistance. The new fourth-generation fluoroquinolones have improved activity against Gram-positive bacterial over their predecessors.
At the same time, there's concern that the fourth-generation fluoroquinolone agents may not be as effective against Gram-negative bacteria as second-generation drugs in this class. It's clinically accepted that the fourth-generation drugs have a broader spectrum of activity than other antibiotic agents and it's their wide-spectrum activity and efficacy that hold the key for their use.
Definite clinical applications exist for the fourth-generation fluoroquinolones -- areas where these new agents really stand out among the other antibiotics we have in our armamentarium. Because they're well tolerated and have a broad spectrum, the fourth-generation fluoroquinolones are a great choice for treating nonspecific acute and perhaps even hyper-acute bacterial conjunctivitis. These agents would also provide the optometrist with reassurance that they're providing adequate prophylaxis for corneal abrasion and erosion therapy.
In this regard, the fourth-generation fluoroquinolones can best be thought of as an "extra powerful and more effective" trimethoprim sulfate/polymyxin B sulfate combination (Polytrim) of the last two decades. The single notable exception is that the FDA approved trimethoprim sulfate/polymyxin B sulfate for use in infants as young as two months.
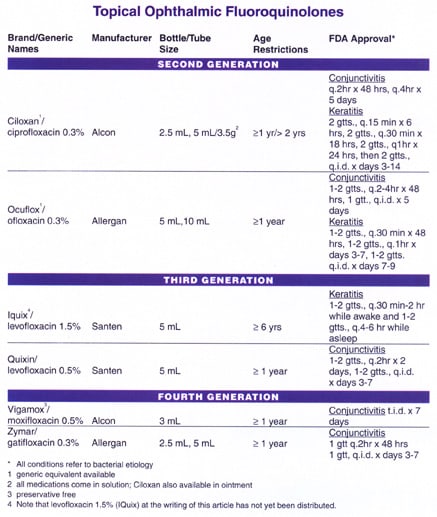
While some of the fluoroquinolones have been tested in younger neonates with good results, all of the fluoroquinolones have a one-year age restriction with the exception of levofloxacin 1.5% (Iquix), which is limited to patients older than six years.
Other similarities with trimethoprim sulfate/polymyxin B sulfate include a broad spectrum of activity and a lack of unwanted side effects. Like trimethoprim sulfate/polymyxin B sulfate, the fourth-generation fluoroquinolones have no reported drug-drug interactions and the only associated contraindication is sensitivity to other drugs in this class.
And finally, like trimethoprim sulfate/polymyxin B sulfate, these agents carry a pregnancy C rating, which means that either animal studies indicate a fetal risk and no controlled studies in women exist or no available studies in women or animals exist. Only consider their use in pregnant women when the benefits outweigh the risk to the fetus.
Another clinical situation where these new antibiotics have been rapidly endorsed is in the pre- and post-op care of cataract and refractive surgery patients (Solomon). In this group of patients, be sure that the antibiotic prophylaxis will cover both Gram-positive and Gram-negative bacteria.
Treating bacterial conditions
Because these fourth-generation fluoroquinolone antibiotics received FDA approval, the question has arisen regarding their efficacy in treating bacterial keratitis. This potentially sight-threatening infection requires antibiosis that's sure to kill any bacteria until laboratory culture identifies the specific infectious etiology. The effectiveness of fourth-generation fluoroquinolones in the treatment of bacterial keratitis is ongoing in therapeutic trials.
Although the initial results may prove promising and while the fourth-generation fluoroquinolones have clear advantages over earlier generation agents, exercise caution with their use in treating bacterial keratitis until the final results of these studies are known and published. The fourth-generation fluoroquinolone agents haven't yet received FDA approval for bacterial keratitis and therefore use in the treatment of this condition remains off-label.
A specific use for fourth-generation fluoroquinolones would be in the treatment of bacterial conjunctivitis in status post trabeculotomy patient. In this setting, we need a fast-acting antibiotic with broad-spectrum coverage because the infectious etiology is unknown.
A drug class to embrace
The new fourth-generation fluoroquinolones bring to our armamentarium an important therapy in treating bacterial conjunctivitis and prophylaxis in corneal injury or pre-/post-surgical care. Their unique "dual mechanism" activity is recognized as a technological advance that holds great promise in limiting the likelihood that bacterial resistance will develop. Patients tolerate these agents well and you can use them safely in a wide range of patients.
References are available on request
Dr. DiMartino is associate professor of clinical optometry at the University of California Berkeley School of Optometry. He's also an associate at Lafayette Optometric Group.
Dr. Whiteside is an assistant clinical professor at the University of California Berkeley School of Optometry. She teaches in the advanced procedures in ocular disease diagnosis laboratory and supervises optometry students in primary care and specialty vision clinics. When she is away from the University, she works in a private practice as well as at Kaiser Permanente.



