Reconsider the risk factors and diagnostic technologies in the management of glaucoma suspects.
Over the past 30 years optometry has been increasingly charged with accurately diagnosing and treating sight-threatening disease. Currently 49 states grant optometrists broader responsibility and prescribing authority than ever before. Increasingly rigorous education and comprehensive standards of care ensure that we provide excellent primary eye care. Therapeutic certification demands we understand the most current and effective treatment options.
Optometrists are uniquely positioned within the health care system to detect emerging ocular diseases such as glaucoma, often before any significant decrease in visual function. However, it can be quite difficult to differentiate patients with early glaucoma from healthy eyes. New risk factors and diagnostic devices have changed our detection criteria. Which glaucoma risk factors should be considered and to what extent they should sway treatment decisions is a contentious topic. We'll discuss these issues here.
Once upon a time . . .
For many years practitioners have based their glaucoma treatment decisions solely on the patients' intraocular pressures (IOP), optic nerve head appearances and/or the status of the visual fields. IOP is no longer considered sufficient to define the presence of glaucoma. Physiologic variations in optic nerve head anatomy in the absence of other known risk factors further complicate decision-making. And finally, visual field performance by even the most capable patients is often inconsistent. When facing patients with these confounding factors, you must question the diagnosis of glaucoma and then proceed with additional testing, as well as more frequent follow-up examinations.
Evaluating the risk for OAG
The overwhelming majority of patients with glaucomatous vision loss have primary open angle glaucoma (OAG). According to the Baltimore Eye Study, approximately 2.2 million Americans have OAG, yet fewer than half of them are aware of it. Unfortunately, marked damage, whether structural or functional, may not be readily evident until later in the course of this slowly progressive process. Following are some additional risk factors to consider in your diagnosis.
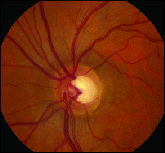
> Age. Recent research indicates that this is a key risk factor for OAG. The Rotterdam Study showed that subjects older than 75 were five times more likely to have bilateral OAG than those younger than 75. Unfortunately, older patients, having been subjected to the disease for a longer duration, frequently present with more advanced damage.
> Race and ethnicity. These are consistent risk factors for the prevalence and age of onset of OAG. Several international studies have shown that glaucoma occurs more frequently and with greater severity within African populations. The OAG prevalence rate in Hispanics lies between that of Caucasians and African-Americans, until later in life when it increases more rapidly than in any other ethnic group.
> Family history of glaucoma. This has important implications for patients. The Rotterdam Study also showed that individuals whose first-degree relatives had glaucoma were about nine times more likely to develop the disease.
> Myopia. It has long been considered a possible risk factor for glaucoma. A 2003 study by Wong et. al showed that myopic eyes were 60% more likely to have glaucoma than emmetropic eyes. More obscure disease associations include low systemic blood pressure, which might diminish ONH perfusion. The relationship between diabetes and glaucoma was discounted in the Ocular Hypertensive Treatment Study (OHTS) study. Links to heart disease, migraine and hypertension are also inconsistent.
> Generous optic disc cupping. This can either be a sign of glaucoma or only a normal, physiologic trait. Determination requires a careful stereoscopic evaluation of the optic nerve head (ONH) on each patient. However, elongation of the cup, particularly vertical, may be the first objective sign of retinal nerve fiber layer (RNFL) loss. Baseline optic disc photos allow for accurate, sequential comparisons. The distribution of RNFL thickness around healthy neural rim tissue often follows the ISNT rule, with the thickest RNFL found inferiorly, followed by the superior, nasal and temporal quadrants. Imaging systems are valuable tools for assessing these and other subtle ONH features.
> Elevated IOP. Whether it's occurring presently, or in the past, elevated IOP constitutes a strong risk for developing OAG. The "normal range" of IOP, derived many years ago, holds that any measurement at or above 22mmHg constitutes ocular hypertension. OHTS concluded that an IOP below 31mmHg by itself is insufficient to diagnose glaucoma.
> Central corneal thickness (CCT). This plays a large role in determining which patients are at greater risk for glaucoma progression. Patients in OHTS with corneal thicknesses greater than 558 microns had a much lower risk of developing glaucoma than those with CCT of less than 555 microns. Unfortunately, it remains unknown whether this relationship is due to corneal hysteresis or other factors such as laminar support.
At our disposal
Automated static threshold perimetry remains a useful tool in the management of glaucoma suspects. Monochromic test strategies are reliable methods of determining functional loss and its progression over time. A depressed pattern deviation suggests pathology that affects the visual system.
Refined psychophysical technologies like frequency doubling perimetry and short wavelength automated perimetry allow earlier detection of subtle disease. As more efficient programs are developed, screening visual fields will become increasingly useful. However, in most practices, formal visual field testing is only ordered when the patient has an increased risk for glaucoma.
Detecting damage sooner
The past several years have seen ONH imaging evolve to where near-histological evaluation of the in vivo posterior pole features is possible. When compared with normal eyes, the RNFL thickness in glaucomatous eyes is significantly thinner, even without detectable visual loss on automated perimetry. Optical coherence tomography (OCT), confocal scanning laser tomography and nerve fiber layer polarimetry each allow fine inspection of RNFL integrity across the neural rim. Quantitative analysis of ONH characteristics provides strong supporting evidence for diagnosing and treating glaucoma by correlating nerve fiber loss to imminent change(s) in visual field.
The Stratus OCT 3000 (Carl Zeiss Meditec) output includes RNFL distribution curves, segmental distribution charts, average thickness parameters and serial comparison charts, which offer a unique diagnostic advantage over traditional examination techniques. A single report that details findings from both eyes makes suspicious anatomic irregularities or asymmetries readily evident. The data arrangement allows convenient comparison of pertinent values on the same test, or over the course of multiple tests. Data presented within this graphical format is easy to understand and facilitates patient education. Ultra-high-resolution optical coherence tomography, currently a research tool, gives extraordinary resolution of posterior structures, promising even finer differentiation of pathologic processes.

|

|
| Analysis
of retinal nerve fiber layer thickness suggests levels at or above normal in all quadrants. |
Significant
loss at the vertical poles consistent with moderately advanced open angle glaucoma. |
Putting it together
A comprehensive eye examination must include a deliberate, systematic evaluation of the patient's risks for developing glaucoma now or in the future. A suitable protocol for every patient with recognizable risk factors would include:
> careful applanation tonometry
> gonioscopy
> stereoscopic evaluation of the optic nerve
> baseline ONH photos
> microanalysis of the RNFL thickness, and
> central corneal pachymetry.
The results gathered from these tests should direct your decisions regarding follow-up intervals, the urgency of automated perimetry testing, and whether to initiate medical therapy or referral for the patient.
Given the strong relationship between CCT and glaucoma risk identified over the past three years, baseline pachymetry has now become standard of care for each glaucoma suspect. And finally, micro-imaging technology, due to its superior sensitivity, may surpass automated perimetry as the most effective ancillary study for making the early diagnosis of glaucoma. OM
References available on request.
| Diagnostic Instruments for Glaucoma By: Andrew S. Gurwood, O.D., F.A.A.O. & Marc D. Myers, O.D., F.A.A.O. Whiile clinical observation remains the mainstay of diagnosing vision-threatening sequellae, recent advances in technology have provided an array of new instrumentation capable of providing data that can improve the detection of subtle abnormalities. These new devices, presented below and in Dr. Guier's article, have the capacity to expose impending, beginning or worsening ocular abnormalities that might otherwise go unmanaged for a period, resulting in permanent disabilities. Heidelberg Retinal Tomograph II (HRT) The HRT confocal scanning laser ophthalmoscope provides a comprehensive analysis of the optic nerve head and peripapillary retina by performing three automatic scans. Each scan is comprised of up to 64 optical sections from the top of the optic nerve head to a depth of 4.0mm, creating a three-dimensionally topographic rendering of the scanned tissue. In glaucoma, three vital structures, the cup, the rim and the retinal nerve fiber layer are examined. Measurements analyze cup shape, depth, area, volume, ratio, retinal nerve fiber layer height and retinal nerve fiber layer thickness. While visual fields provide an overview of retinal function, the HRT gathers structural data. This allows you to follow subtle changes in tissue morphology over time. It's an excellent method of surveying and correlating tissue damage with functional loss. It may also serve as a modality for early detection of worsening disease. GDxVCC Nerve Fiber Analyzer The GDX is a scanning laser polarimeter, which passes polarized light through the nerve fiber layer. It measures the shift in the light's polarization, or retardation. The measured difference between the initial signal and the recaptured signal can be calculated to measure the nerve fiber layer thickness. The variable corneal compensator feature determines and corrects for each patient's non-retinal nerve fiber layer retardation differences (mostly produced from the effects of the cornea). After a measurement is taken, the patient's retinal nerve fiber layer is automatically compared with a large normative database, allowing the examiner to identify potential areas of structural compromise. The GDX may be particularly useful in the diagnosis and management of glaucoma, although the instrument is not specifically dedicated to only that disease. The testing procedure takes only seconds to complete and possesses excellent reproducibility. The procedure requires no pupillary dilation and minimal operator training. References available upon request. |



