CASE STUDY
Sir, You Look Pale
A senior citizen reports sudden painless and almost complete vision loss upon waking.

Andrew S. Gurwood, O.D., F.A.A.O.
Andrew S. Gurwood, O.D., F.A.A.O., Dipl., Philadelphia, Pa.
A 64-year-old black man pres-ented to the office via the emergency room. His chief complaint: a painless and almost complete loss of vision OS. He explained to the emergency room physicians at 10:00 a.m. that morning that he went to sleep with his vision intact the night before, but awoke at 6:00 a.m. with a large, persistent black spot preventing vision in his left eye.
The emergency-room physicians uncovered a history of poorly controlled hypertension and chronic cocaine abuse by way of inhalation (snorting). The patient denied any previous trauma or allergies, and his past history revealed no other medical or ocular abnormalities. Further, his past family medical histories were unremarkable.
Exam findings
The patient's best-uncorrected visual acuities measured 20/20 OD and hand motion (HM) OS. He had a grade IV afferent papillary defect.
Confrontational visual fields uncovered no light perception (NLP) vision in all but the most temporal peripheral portions of the left visual field. The right eye was normal.
Color testing and brightness testing were equally compromised OS. Extraocular muscles were normal OS. Refraction uncovered a clinical emmetropia with clear retinoscopic reflexes OS.
Biomicroscopy with gonioscopy revealed normal anterior segment structures, normal anterior chamber angle structures and normal anterior vitreous humor with no evidence of primary angle closure, secondary angle closure, plateau iris, pseudoexfoliation, pigment dispersion or angle neovascularization in both eyes.
Tonometry measured 12 mm Hg, OU. The dilated fundus examination demonstrated the classic, signature appearance of a central retinal artery occlusion (CRAO) (attenuated retinal arteriolar vasculature and retinal pallor, usually including a cherry-red spot in the fovea), without macular sparing, OS.
Diagnosis
I diagnosed this patient with CRAO.
Discussion
In 1859, German ophthalmologist Albrecht Von Graefe described an embolic event to the central retinal artery in an endocarditis patient. Practitioners would later refer to this event as central retinal artery occlusion (CRAO).1
CRAO results when the main artery that supplies blood to the retina becomes blocked, causing sudden vision loss. The hallmark symptom of acute CRAO: abrupt, painless vision loss. Pain is unusual and suggests associated ocular ischemic syndrome. Amaurosis fugax precedes visual loss in about 10% of patients.2 Rarely, in cases associated with arterial spasm, a relapsing and remitting course of visual loss precedes CRAO.2,3
Examination of CRAO patients typically reveals a visual acuity of 20/800 or worse.1-3 HM- or light-perception vision can occur, but NLP vision is uncommon, except in the setting of an ophthalmic artery obstruction or temporal arteritis. If a patent cilioretinal artery is present and perfuses the fovea, normal central acuity may be present. An afferent pupillary defect on the affected side is the rule.1-5 In the acute stages of CRAO, anterior segment examination appears normal. The exception: In the setting of concurrent ocular ischemic syndrome, you can see neovascularization of the iris.
Within the first few minutes to hours after the obstruction, the fundus may appear relatively normal. Eventually, the decreased blood flow results in ischemic whitening of the retina in the territory of the obstructed artery. A cherry red spot of the macula is typical and a thin nerve fiber layer allowing visibility of the underlying choriocapillaris in contrast to the surrounding white, ischemic retina causes it.
Pallid swelling with splinter retinal hemorrhages signals what's known as "a choked optic disc," meaning it's struggling for oxygen. Differential diagnoses include mild, nonischemic central retinal vein occlusion and lipid with hemorrhage indicating hypertensive retinopathy or neuroretinitis.
After four to six weeks, the retinal whitening dissipates, leaving the optic disc to develop pallor. Arterial collaterals may also form about the optic disc. No foveolar light reflex is apparent and because of the stress to the retinal pigment epithelium, fine clumped hyperplasia may develop.2 Secondary ocular neovascularization isn't uncommon after CRAO. Iris neovascularization occurs in 18% of patients, with many of these eyes going on to develop neovascular glaucoma.2,6 When you discover neovascularization, make a referral to a retinologist for panretinal laser photocoagulation to reduce the risk of neovascular glaucoma.2,6
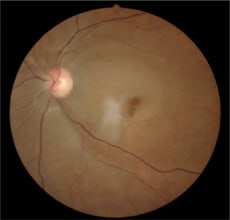
Pallid retinal edema is consistent with CRAO, seen here in the patient's left eye.
Researchers currently believe that thrombus formation at or just proximal to the lamina cribrosa causes the majority of central retinal artery obstructions.2-4 Atherosclerosis is implicated as the most common inciting event of thromboembolitic debris.2 Meanwhile, cocaine-induced arterial thrombosis is rare. Most reported cases involve small-diameter vessels, such as the cerebral and coronary arteries.4,5
Other CRAO causes:
► Blood conditions. Coagulopathies or poor blood flow secondary to proximal arterial disease has been associated with both central- and branch retinal artery obstructions.2 Further, blood clotting abnormalities, such as antiphospholipid antibody syndrome, protein S deficiency, protein C deficiency and antithrombin III deficiency are associated with CRAO.1-5
► Systemic disease. About 60% of CRAO patients have concurrent systemic arterial hypertension, while diabetes is present in 25%. Although patients who suffer from retinal artery obstruction commonly have systemic diseases, the true cause and effect may not be clear (In this case, circumstantial evidence points to substance abuse and hypertension as factors.) Systemic evaluations in what practitioners thought to be otherwise healthy individuals reveals no definitive cause for the obstruction in more than 50% of affected patients. Further, investigators find potential embolic sources in less than 40% of cases.2,6,7
► Heart disease. Hemodynamically significant ipsilateral carotid artery disease is present in about one third of affected patients.1,2 An embolic source from the heart is present in less than 10% of CRAO patients.2 Still, you should refer CRAO patients, especially patients younger than age 50, for echocardiography because this group of individuals is less likely to suffer from significant atherosclerotic disease and more likely to have an inherent mechanical issue which produces plaques. Further, a cardiologist may deem a transesophageal echocardiography necessary to reveal deeper cardiac embolic sources. This test examines the heart's valves through the esophagus and can provide an excellent study of heart anatomy and function.2,6,7 Although temporal arteritis, a devastating and sometimes lifethreatening arterial inflammation, is present in less than 5% of CRAO cases, you must rule it out in all patients older than age 50.1-5
► Optic neuritis, or even orbital disease, such as mucormycosis.2
► Local trauma producing damage to the optic nerve or blood vessels may lead to central retinal artery obstruction, arterial spasm or dissection.2
► Exposure to radiation.2
► Behcet disease.1
► Migraine.1
► Syphilis.1
► Optic disc drusen.2
► Prepapillary arterial loops.2
CRAO diagnosis is straight-forward when diffuse ischemic retinal whitening is present in the setting of abrupt, painless visual loss. Fluorescein angiography may help if the diagnosis is unclear. A delayed arm-to-retina circulation time with a leading edge of dye visible in the retinal arteries is characteristic for CRAO.2,6,7,3
Electroretinography characteristically reveals a decreased to absent b-wave with intact awave. Visual fields often show a remaining temporal island of peripheral vision. If a patent cilioretinal artery is present, a small intact central island of vision corresponding to the perfused retina is present as well.2
Color Doppler imaging (ultrasonography) can help to determine the blood flow characteristics of retrobulbar circulation. You can also use it to detect calcific sources of thrombo-emboli at the lamina cribrosa and to monitor blood flow changes induced by therapy (discussed below). You should order carotid artery studies, consistent with a vascular investigation.2,6,7
CRAO is a rare event. Sources estimate its occurrence at one in 10,000 outpatient visits to the eyecare professional.2 Men are affected more commonly than women by the ratio of 2:1, with a mean age of onset of about age 60. Bilateral involvement occurs in 1% to 2% of cases.2,6,7,3
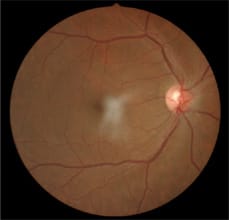
The 64-year-old patient's right eye, which appeared normal.
Clinically speaking, only 20% to 25% of cases demonstrate visible emboli in the central retinal artery or one of its branches.1,2 The site of obstruction may or may not be visible upon clinical examination.
I've found that if an obstruction exists for more than 100 minutes, complete irreversible death of the inner retina occurs. In practice, it's rare when a patient who has experienced a severe visual disability experiences total spontaneous recovery. However, such cases do exist, even after several days of visual loss. Spontaneous recovery may be more common in children.2
Management
I ordered laboratory testing to rule out coagulopathy, hyperviscosity, cardiac etiology, infection, inflammation, space-occupying lesions and demyelination. The studies included complete blood count with differential and platelets, blood pressure, fasting blood sugar, lipid panel, cholesterol, erythrocyte sedimentation rate, c-reactive protein, human leukocyte antigen test (HLA - B27), fluorescent treponemal antibody absorption test (FTA–Abs), reactive plasma reagin test (RPR), human immunodeficiency virus titre (HIV titre), electrocardiogram with 2-D echo, a transesophageal electrocardiogram, carotid doppler and magnetic resonance imaging. (Additional tests may include blood pressure measurement, ophthalmodynamometry and carotid auscultation.)
No proved treatment exists for CRAO, but treatment strategies center on the following goals:
► increasing retinal oxygenation,
► increasing retinal arterial blood flow,
► reversing arterial obstruction,
► preventing hypoxic retinal damage.
You can best preserve retinal survival in these cases when you address the underlying embolic pathology in less than 100 minutes via topical and oral medications or paracentesis to lower the patient's intraocular pressure (IOP). This, in turn, decreases retinal blood flow resistance, which increases retinal arterial blood flow, moving the embolus further down the arterial tree.2
| CRAO ASSOCIATED WITH TEMPORAL ARTERITIS REQUIRES EMERGENT HIGH-DOSE CORTICOSTEROID TREATMENT. |
Given the time that had elapsed between the event and the patient's presentation to the office, the attending ophthalmologist made the decision that the risks of surgical intervention outweighed what he deemed to be of little benefit.
After querying the patient for contraindications, I administered a 350mg tablet of aspirin for anticoagulation purposes. While researchers have reported that fibrinolytic medication use may have some promise, controlled clinical trials have been designed to test this hypothesis.2 Anecdotal reports of success using heparin, tissue plasminogen activator, streptokinase and urokinase do exist.2 Also, some researchers have reported success in select cases using an intra-arterial injection of tissue plasminogen activator, streptokinase or urokinase.8,9
I performed aggressive digital ocular massage in an attempt to stimulate autoregulatory retinal mechanisms, which may increase retinal arterial size, allowing the plaque to become dislodged. I simultaneously administered ocular anti-hypertensive medications (topical beta blocker, topical iopidine and oral acetazolamide [Diamox, Lederle Laboratories] 2 x 250mg tablets in an attempt to lower the patient's IOP to decrease resistance to nerve and retinal blood flow, increasing the pressure head to raise perfusion and possibly push the plaque ahead. While research has shown that these and other modalities, such as sublingual nitroglycerin, pentoxifylline [oxpentifylline], calcium channel blockers and b-blockers are rarely efficacious, some practitioners use them in an attempt to stave off certain catastrophic vision loss.1
Finally, I attempted additional retinal autoregulatory stimulation via oxygen deprivation by having the patient breath into a brown-paper-bag. The preparation carbogen (95% oxygen, 5% carbon dioxide) also can stimulate the retinal arterial changes associated with decreased oxygenation, however, anecdotally, this too has limited success.
CRAO associated with temporal arteritis requires emergent high-dose corticosteroid treatment.1-3 Without therapy, the risk to the second eye and to the individual is great. Although the first-affected eye rarely recovers, visual recovery can occur from CRAO associated with temporal arteritis in instances in which you administer high-dose intravenous methylprednisolone.2,3
Although the patient's IOP dropped to under 8mm Hg OU, he reported no reversal of symptoms. Therefore, I educated him about his diagnosis, and I informed him that his vision loss would most likely be permanent. I also instructed him to obtain all the studies initially ordered and to follow through with the retinology referral we'd (the ophthalmologist and I) made. Further, I asked him to return to a neuroophthalmologist.
I referred the patient to a neuroophthalmologist and retinologist to confirm my diagnosis and ensure the patient didn't have any other critical central nervous system issues, such as giant cell arteritis, that would require therapy or additional testing. The neuroophthalmologist saw the patient within four hours, while the retinologist saw him at the end of that day. Both concurred with my diagnosis, which was ultimately presumed to be secondary to emboli precipitated by chronic substance abuse.
I last saw this patient at this initial visit. I placed his care in the hands of the experts (the neuroophthalmologist and retinologist) who would refer him back to me for advice on referrals to appropriate services for the blind or vision rehabilitation.
Because I've not received any further information (his test results, for instance,) I am led to believe that the patient has yet to return to these specialists for follow-up. OM
references
1. emedicine. Central Retinal Artery Occlusion. Graham RH. www.emedicine.com/oph/topic387.htm (accessed 11/01/07)
2. Duker, J.S. Retinal Artery Obstruction. In : Yanoff, M., Duker, J.S. Ophthalmology. Philadelphia, PA, Mosby 1999;8(17):17.1-17 . 17.8.
3. Chan CC, Paine M, O'Day J. Steroid management in giant cell arteritis. Br J Ophthalmol. 2001 Sep;85(9): 1061-4.
4. Zhou W, Lin PH, Bush RL et al. Acute arterial thrombosis associated with cocaine abuse. J Vasc Surg. 2004 Aug;40(2):291-5.
5. Michaelides M, Larkin G. Cocaine-associated central retinal artery occlusion in a young man. Eye. 2002 Nov;16(6): 790-792
6. Ko, MK, Kim, DS. Posterior segment neovascularization associated with acute ophthalmic artery obstruction. Retina. 2000;20(4): 384-8.
7. van Norel, J, van den Biesen, PR, Groen, GJ, van Norren, D. Hold up of dye in the arm during fluorescein angiography: a quantitative demonstration. Am J Ophthalmol. 2000 Apr;129(4):551-2.
8. Schmidt DP, Schulte-Mönting J, Schumacher M. Prognosis of central retinal artery occlusion: local intraarterial fibrinolysis versus conservative treatment. AJNR Am J Neuroradiol. 2002 Sep;23 (8):1301-7.
9. Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999 Dec;128(6):733-8. Erratum in: Am J Ophthalmol 2000 Dec;130(6):908. OM
Dr. Gurwood is a professor of Clinical Sciences at Pennsylvania College of Optometry. He practices at the Albert Einstein Medical Center's Department of Ophthalmology in Philadelphia. E-mail him at agurwood@pco.edu.



