DIAGNOSTICS
Quantify RV Diameter
Assess the risk of certain ocular problems
and systemic diseases via retinal vessel diameter.
By
Raymond Wyant, David Kairys, O.D., M.S., Harold Webster, Ph.D.
|
|
|
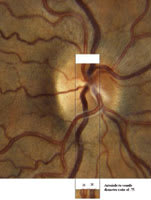
Figure 1: Arteriole = 20. Venule = 15. AV Ratio = 0.75. |
Retinal arteriolar and venular lumen width can reflect vascular health and yield some degree of risk for the future develop-ment of certain ocular problems, such as glaucoma, and systemic diseases, such as heart disease.1,2 We use the term "SAHARA" test to encapsulate a clinical phenom-enon that is widely recognized within the ophthalmic community: namely that "Skinny Arterioles and Heart Attack Risk" are correlated. (See "The Link Between Retinal Vascular Anatomy and Ocular and Systemic Conditions," page 31.) Therefore, you could use a test that can reliably quantify retinal arteriole-to-ven-ule diameter (A/V) ratio to ensure you identify all patients at risk for these conditions.
Currently, you can make qualitative estimates of retinal vascular integrity by recording the A/V ratio. However, this method limits your ability to
reliably quantify A/V for two reasons:
• The patient's A/V ratio can be due to either narrowed arterioles and/or venules that are too dilated, and there is no readily accessible means to determine which is the root cause.
• The recording of A/V ratio is essentially subjective in nature, as you may have a different anat-omic appreciation of the retinal vasculature than another O.D. (inter-observer variation). Also, your estimation of internal ocular structures, such as cup/disc, size of disc and A/V ratio can vary from day-to-day (intra-observer variation).
Thus far, access to expensive and complex photographic and computer-driven techniques have allowed practitioners only in tertiary-care research settings to achieve quantitative measure-ments of vessel lumen diameter.
|
|
|
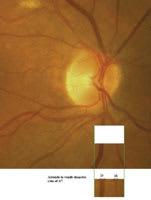
Figure 2: Arteriole = 18. Venule = 25. A/V Ratio = 0.72. |
But this is about to change, as we developed a simple, inexpensive and easily accessible method for you, the primary-care O.D., to measure A/V quantitatively. This method was the brainchild of a college freshman (Ray Wy-ant), who was challenged to invent a repeatable quantitation technique based on the current medical literature and evolving knowledge about the topic of vessel lumen diameter.
Here, we will describe how you can master this method, how it was successfully used and what the future holds for it.
Master the method
Before you can master this method, you will need the following materials:
• An off-the-shelf 4.0 mega-pixel digital camera with the least resolution you can tolerate.
• Either a PC or Mac made within the last two years, as these computers have Photoshop capability (memory and speed).
• A computer printer adaptable to that computer.
• Microsoft Paint software you can obtain at no charge via the Internet. (Use your favorite search engine to obtain this software.)
• A 90.00D diagnostic lens.
|
|
|
Figure 3: Arteriole = 21. Venule = 28. A/V Ratio = 0.75. |
Now, position the patient on a standard slit-lamp biomicroscope, and use a standard 90.00D diagnostic lens to visualize the optic nerve head. Once you identify several pairs of arterioles and venules, focus the microscope on one of the side-by- side pairings. Line the camera with one of the biomicroscope oculars, and take one photo (see figure 1).
Import the image into your computer and use the Microsoft Paint software to view the image on your screen. Also, use the Paint software to isolate a small (1/20 Disc diameter sized) area of the photo, where the two vessels run parallel. The purpose: Other researchers have deter-mined their maximum utility in comparing arterioles with ven-ules is in the area surrounding the disc within two disc
diameters (4mm). The narrowing down to a 1/20 disc diameter size allows you to specifically lay down marking pixels on specific vessels. This consistency allows for true, confident comparison.
Now, use the Paint program's zoom feature to magnify the image, and locate the outer most edges of the arterioles and venules. Choose diameter this way to reflect the true anatomic relationship. One could argue that the pixels would more accurately reflect cardiovascular status if we were to measure only the lumen and exclude the walls of the vessels. Now, mark these outer edges with a box via the Paint program's smallest-sized electronic paintbrush.
Make one dot every other pixel with the Paint program's pencil tool (see figures 2 and 3). Now, convert that area of the photo to black-and-white for better contrast (see figure 4), and print the slightly out-of-focus photo onto standard, store-brand photocopy paper. (Use the slightly out-of-focus photo to control cost. An 8-megapixel camera with high-resolution would improve your ability to appreciate other anatomic as-pects of the retinal microvasculature, but it would have no im- pact upon the measurement at hand.)
|
|
|
Figure 4: Arteriole = 24. Venule = 16. A/V Ratio = 0.67. |
Count the marks and divide the arteriole diameter (in pixels) by the venule diameter (in pixels), and you achieve the final ratio of diameters.
We first used this method on a healthy, 30-year-old patient who presented for her regular exam and volunteered to be our Guin-ea pig. She had no family or personal history of cardiovascular disease, migraines, hypertension, diabetes or cigarette use. This patient's exact A/V ratio measure was 0.708 (A=8.5 dots and V=12 dots). There is as yet no universally accepted ratio to predict vascular disease, however, the community consensus holds A/V of 0.33 as a risk factor and A/V 0.67 as a protective factor. Thus, we deemed our 30-year-old patient healthy. The actual time spent positioning the patient and counting pixels took about three minutes.
The future
We realize many of you will not be motivated to undertake the same kind of effort to use this method, however minimal, due to time constraints.
That is why we hope to develop a prefabricated — ideally hand-held and battery-operated — instrument you can purchase from an equipment supplier. The instrument would resemble a standard ophthalmoscope with an added component that would house a digital camera, a dedicated computer, a closed-circuit TV LCD viewing screen and a USB port.
|
THE LINK BETWEEN RETINAL VASCULAR ANATOMY AND OCULAR AND SYSTEMIC CONDITIONS |
|
Because
retinal vascular anatomy correlates with certain ocular conditions and systemic
physiologic diseases, you, the primary-care physician, may be the first practitioner
to identify a life-altering condition in a patient. For this reason, you should
be aware of the various ocular manifestations that can signal these conditions. For example, the identification of intraocular flame-shaped hemorrhages is often a sign of high blood pressure. In addition, dot-and-blot hemorrhages, seen via direct ophthalmoscope, are usually characteristic of diabetes.3,4 More recently, researchers have linked changes in retinal blood vessels with arteriolar-sclerosis, diabetes, increased likelihood of heart disease and even increased probability of developing glaucoma.1-5 Indeed, arteriolar narrowing observed in the area of the retina just beside the optic nerve head has been correlated with similar narrowing of the arterioles of the cardiac muscle and hence, with increased incidence of heart attack.2 While this so-called "SAHARA" phenomenon has been widely recognized within the ophthalmic community, it has only recently been experimentally documented and verified.2 People who have smaller arteriolar diameters are more likely to experience myocardial infarctions as well as stroke and even migraine.2 De-creased arteriolar diameter also predisposes patients toward the development of age-related macular degeneration, presumably via impairment of retinal perfusion.6,7 Carbon Monoxide (CO) poisoning and systemic inflammatory diseases, such as diabetes, cause retinal venules to dilate so that you may be able to initiate or complement a medical diagnosis based on a simple, quick and noninvasive procedure (ophthalmoscopic observation and subjective impression constitute the standard methodology).8 Further, when the venule caliber expands just beside the optic nerve head, researchers have observed that blood also contains certain chemicals correlated with inflammation and hence increased risk of sequelae of those inflammatory entities.9 Researchers have associated deviations of posterior pole arterioles and venules from "normal" calibers with several systematic disease entities. For example, narrowing of the arteriolar lumen diameter can result from high blood pressure as well as hyperlipidemia, and it has been correlated with progression from ocular hypertension to glaucoma.1 Putatively, smaller arteriolar diameter makes the optic nerve head more susceptible to diurnal variations in the intraocular pressure and so leads to a sequence of biomechanical and chemical events that eventuate in death of neurons in the optic disc.8 |
With the ocular component of the device, you would place an illumination spot upon a vessel pair, within 1 disc diameter of the edge of the nerve head. Then, after snapping a picture that is captured by the device's software, you would move to a subsequently observed vessel pairing. While focused upon each pairing, you would take a photo, which would automatically be uploaded to the computer for a quick A/V reading to be displayed on the view screen. As you photograph each succeeding A/V pairing, the computer would calculate the mean A/V and standard deviation of the samples taken from that mean. Both eyes could be sampled within two minutes, yielding up to a dozen A/V readings, and therefore, a statistically valuable index of vascular health status.
By educating yourself on the correlation between retinal vascular anatomy and ocular and systemic physiologic conditions and implementing the afore-mentioned method of reliably quantifying retinal A/V, you play a vital role in seeing that your pa-tients gain access to appropriate medical services, such as cardiovascular workups and glaucoma testing. This will ensure patients ultimately get the care and/or treatments they need to ensure their quality of life.
1. Cohen, SL, Lee PP, Herndon LW, et.al. Using the arteriolar Pressure Attenuation Index to predict ocular hypertension progression to open-angle glaucoma. Arch Ophthalmol. 2003 Jan;121(1):33-8.
2. Wong, TY, Klein R, Sharett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002 Mar 6;287(9):1153-9.
3. Owen CG, Newsom RS, Rudnicka AR, et al. Vascular response of the bulbar conjunctiva to diabetes and elevated blood pressure. Ophthalmology. 2005 Oct;112(10):1801-8.
4. Barile, GR. Current Ophthalmology and Medicine. Survey of Ophthalmology. 2005 June 50(6): 607-608.
5. Stefanson, E, Landers MB, Wolbarsht M. Retinal vessel caliber and diabetic retinopathy. Arch Ophthalmol. 2005 May;123(5):709.
6. Donoso LA, Kim D, Frost A, et al. The role of inflammation in the path-ogenesis of age-related macular degeneration. Surv Ophthalmol. 2006 Mar-Apr; 51(2):137-52.
7. Wangsa-Wirawan ND, Linsenmeier, RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003 Apr;121(4):547-57.
8. Weigert G, Findl O, Luksch A, et al. Effects of moderate changes in intra-ocular pressure on ocular hemodynamics in patients with primary open-angle glaucoma and healthy controls. Ophthalmology. 2005 Aug;112(8):1337-42.
9. Klein, R, Klein BE, Knudtson MD, et.al. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006 Jan;124(1):87-94.

Mr. Wyant is a member of the National Honor Society and is presently an undergraduate student at Pennsylvania State University.
Dr. Webster is a tenured professor of biological sciences at Pennsylvania State University. He has previously authored two other optometric-themed articles with Dr. Kairys. One was on tear chemistry, and the other was about a rare pediatric eyelid infection.