LEADING OFF
TIPS, TRENDS & NEWS YOU CAN USE
FDA GREEN LIGHTS XIIDRA FOR DRY EYE DISEASE SIGNS AND SYMPTOMS
The FDA recently approved lifitegrast ophthalmic solution 5% (Xiidra, Shire), indicated for the treatment of dry eye disease (DED) in adult patients.
Xiidra is a lymphocyte function-associated antigen 1 antagonist, the first prescription eye drop that is FDA-approved to treat both the signs and symptoms of DED. Shire expects to launch the prescription eye drop in the third quarter of this year. It is dosed b.i.d., in each eye.
“. . . We’ve done an extensive amount of research both with consumers as well as eye care professionals, and it really is the symptoms of dry eye disease that drives these patients into their doctor’s office. . .” explains Robert Dempsey, vice president and head, Ophthalmics, Shire.
He says Xiidra, which got its name when the double “i” was deemed “eye catching” by Shire, is the company’s first approval in ophthalmics. Shire is also investigating therapies for infectious conjunctivitis, retinopathy of prematurity, autosomal dominant retinitis pigmentosa and glaucoma as well.
“We’re building a comprehensive franchise in the anterior segment, the posterior segment and in inherited retinal disease,” Mr. Dempsey says. ■
OPTOMETRIST READIES U.S. WOMEN’S SOCCER TEAM FOR RIO
With the 2016 Olympic Games starting this month, the 2015 FIFA World Cup Champion U.S. Women’s National Soccer Team (USWNT) has been hard at work on fitness, strategy, mental preparedness and their vision. Bronson Hamada, O.D., of Surf City Optometry, in Huntington Beach, Calif., oversees the latter.
“Almost every member of the team has 20/12+ or better visual acuity, which puts them in the 97th percentile of visual resolution. But, even at the elite level, approximately 18% to 20% of athletes have some sort of visual deficit that they are not aware of,” explains Dr. Hamada.
“My job is to identify and correct small refractive errors, decrease anisometropia, correct minimal amounts of cylinder, provide lenses that have better optics, comfort or stability of clarity and provide vision training services. . .” he says. ■
ALLERGAN FILES FDA APPLICATION FOR OCULEVE
Allergan has submitted a de novo FDA application for the approval of its Oculeve Intranasal Tear Neurostimulator. This type of application is for new devices that have reasonable assurances of safety and effectiveness.
Oculeve is a handheld device that stimulates tear production in patients who have dry eye disease by sending micro-electrical pulses to the lacrimal gland. In May, Allergan revealed that two pivotal trials of the device, OCUN-009 and OCUN-010, showed increases in tear production, as measured by Schirmer score, meeting their primary and secondary efficacy endpoints. ■
3 REASONS
TO VISIT THE OM WEBSITE
1 Search complete issue archives of Optometric Management.
2 Read (and subscribe to) OM Newsletters, including the “Management Tip of the Week.”
3 Access webinars, videos and other exclusive content.
THREE CONTACT LENSES INTRODUCED AT OPTOMETRY’S MEETING
Three contact lens companies unveiled new offerings at Optometry’s Meeting:
• DAILIES TOTAL1 Multifocal (Alcon). The daily lens, designed for people with presyopia, has water gradient technology, Delefilcon A material, a Dk/t of 156 @ -3.00D, a 14.1mm diameter, an 8.5mm base curve and powers of -6.00D to +3.00D (in 0.25 steps), with “LO, MED, and HI” add powers. Availability: now.
• Biofinity Energys (Cooper-Vision). Designed for digital device users, this monthly replacement lens has Digital Zone Optics, Aquaform Technology, Comfilcon A material, a Dk/t of 160 (at -3.00D), a 14.0mm diameter in sphere powers from +8.00 to -12.00, a 8.6mm base curve with an 0.08mm center thickness at -3.00D and powers of +8.00 to -12.00. Full availability: fall.
• ACUVUE VITA (Johnson & Johnson Vision Care). This monthly lens features HydraMax technology, an Infinity Edge, Senofilcon C material, a Dk/t of 147 for –3.00D, a 14.0mm diameter and 8.4mm/8.8mm base curves. The powers are –0.50D to –6.00D in 0.25D steps, –6.50D to –12.00D in 0.50D steps, +0.50D to +6.00D in 0.25D steps, +6.50D to +8.00D in 0.50D steps. Availability: now. ■
Optometric Management will provide individual coverage of each lens in upcoming “Contact Lens Focus” departments.
MEN, WEALTHY AND YOUNG USE MVC MOST, REVEALS REPORT
Most adults who use their managed vision care (MVC) plans are likely to be male, high-income buyers and younger than age 45. They also are more apt to buy lenses from an independent doctor, all according to The Vision Council’s continuous research study, the VisionWatch report.
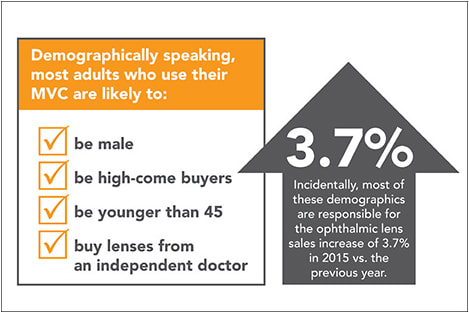
Courtesy The Vision Council
The primary reason why men, higher-income people and adults younger than age 45 are using insurance benefits more than other demographics is that they “are more likely to either have a current full-time job that offers vision insurance/MVC benefits or receive those MVC/vision insurance benefits from someone else currently employed and receiving family vision insurance benefits,” explains Jessica Lutz, a spokesperson for The Vision Council. “That higher level of coverage among certain demographics corresponds to a higher level of utilization when making eyewear purchases with insurance benefits.”
In terms of “why” those with MVC benefits often turn to independent ECPs for eyewear and eye care needs, Ms. Lutz says they tend to be a more “medically oriented” customer. ■
Research Notes
• A noninvasive, direct electric current to the visual cortex for 20 minutes can immediately improve VA in the parafoveal belt, and those with the poorest acuity benefitted the most, reveals June’s Current Biology ■
• Latanoprostene Bunod ophthalmic solution 0.024% for open-angle glaucoma or ocular hypertension provided P≤0.025 IOP reduction when used once at bedtime for three months at all but week 2 (subjects were evaluated nine times), reveals August’s American Journal of Ophthalmology ■
• Prior intensive glycemic control decreased diabetic retinopathy risk by 50% in Type 2 patients four years after undergoing fenofibrate treatment, though the drug’s benefit and intensive blood pressure control had no effect, reveals July’s Diabetes Care ■
• Bevacizumab (Avastin, Genentech) and ranibizumab (Lucentis, Genentech) were deemed safe and effective for AMD treatment, but 20% of eyes declined to 20/200 after two years, says the May 2 online edition of Ophthalmology ■
• Indiana University researchers have created a virtual tissue model of diabetes in the eye. The results, reported in the journal PLOS Computational Biology, show precisely how a small protein that can both damage or grow blood vessels in the eye causes vision loss and blindness in people with diabetes ■
CAROTENOID SUPPLEMENTS CAN IMPROVE CONTRAST SENSITIVITY, REVEALS STUDY
Carotenoid supplementation lead to statistically significant increases in contrast sensitivity at 1.2cpd and 6.0cpd in healthy eyes, reveals June’s Investigative Ophthalmology & Visual Science. The study’s researchers say this supplementation could be particularly beneficial to athletes and members of the military, as both must quickly identify fast-moving or small objects.
The double-blind, placebo-controlled block-randomized study was comprised of 105 subjects ages 20 to 60 who received the placebo (52 subjects) or the supplement (10mg lutein, 2mg zeaxanthin and 10mg meso-zeaxanthin) (53 subjects). Contrast sensitivity was assessed at baseline, three months, six months and one year.
The study’s purpose was to determine the effects of macular carotenoid supplementation on visual function in normal subjects with low macular pigmentation. A second will focus on early AMD. ■

■ “To the victor, goes the spoils!”: The Michigan College of Optometry at Ferris State University celebrates its win of Essilor’s Varilux Optometry Student Bowl. Essilor also awarded the college with $1,000. ■



