Making the diagnosis from glaucoma suspect to glaucoma and predicting disease progression can be challenging for the clinician. Fortunately, our diagnostic tool box continues to expand with new technology. However, nothing replaces the doctor, thankfully.
Here, I discuss what, specifically, requires evaluation and the tools that can aid in these evaluations.
PUPIL ASSESSMENT
Glaucoma is a disease of the optic nerve and, pupillary assessment determines its function. For many, the presentation of primary open-angle glaucoma (POAG) is asymmetric and can cause a relative afferent pupillary defect (RAPD). A sluggish or ovoid pupil can alert the practitioner to evaluate the angle and iris for pigmentary dispersion, narrow angles, synechia and neovascularization.
Tools to aid in evaluation. The swinging flashlight test, RAPDx (Konan Medical), APD Tester (Good-Lite Co.) and ColorDx CCT-HD (Konan Medical).
The swinging flashlight test — with the tried-and-true transilluminator — remains the standard of care to measure RAPD. One can utilize neutral density filters to grade the severity. Neutral density filters are available in a variety of densities, with 0.3, 0.6, 0.9 and 1.2 log units being most helpful in grading an RAPD. The RAPD can be graded by holding the neutral density filter over the good eye to quantify the level of the defect.
The APD Tester aids in the diagnosis of RAPD by allowing for the simultaneous viewing of the pupils.
The RAPDx is an infrared video pupillography device that allows for an objective pupil assessment. Studies show pupillography is useful in discriminating persons with glaucoma from those who have normal eye exams.1
The ColorDx CCT-HD, a self-administered test with randomized test plates, looks for protan, deutan and tritan deficits and measures the severity. Testing for acquired color deficiencies is widely underutilized, but can be seen in glaucoma progression.2 Past research shows blue-yellow defect in glaucoma. A recent abstract by Milton Hom, O.D., shows this color vision test correlates with both visual field (VF) defects and ganglion cell complex thinning.
IOP
Understanding this measurement and its variability is essential for choosing an effective target pressure and guiding treatment choice. Too often, we rush to treat based on one elevated IOP reading. Personally, unless this reading is 30 mmHg or higher, putting the patient at risk for vaso-occlusive events, gathering more data is helpful for these patient’s long-term management. Therefore, prompt return for serial IOP evaluation and further testing is recommended. Many factors influence accurate IOP measurement, including the methods used to attain the reading, a patient’s central corneal thickness (CCT) [an independent risk factor of glaucoma, according to the Ocular Hypertension Treatment Study (OHTS)] and corneal hysteresis (CH). Non-contact tonometry continues to be a fast and easy method for IOP determination, however this is less accurate than applanation tonometry. Our understanding of corneal dynamics continues to influence interpretation of the IOP reading, aid in our ability to predict risk for progression and adapt treatment accordingly.
Tools to aid in evaluation. Tonometry, pachymetry and CH.
Goldman applanation tonometry remains the gold standard and most widely used method for measuring IOP, yet more recent tonometers, such as those that take additional measurements, are revolutionizing IOP measurement. Factors can influence this measurement, including, tight lid, holding the eyelid for applanation and placing pressure on the globe inadvertently and utilizing too much or too little fluorescein sodium and benoxinate hydrochloride solution for the measurement.
The Icare HOME tonometer (Icare USA) allows for 24-hour IOP monitoring outside normal clinic hours, as fluctuation in the IOP can be thought of as a diurnal variation occurring during a single day, short-term fluctuation that is occurring through days and weeks and long-term fluctuation occurring through months and years.3 Data garnered from the device may help identify IOP variations and spikes that the patient may experience outside the office.
Pachymetry can aid in determining CCT value, which is important because it can create falsely high IOP. OHTS introduced the idea of IOP recalculation based on CCT, and some EHRs have even been programmed to create this new IOP once the pachymetry is entered in to the patient’s chart. Through time, the understanding of CCT has evolved, and it is no longer standard practice to recalculate the IOP based on this reading. It is, however, used as a risk measure. For patients with thinner than average CCT, < 555μm, there is an added risk for progression to glaucoma, while those who have thicker than average CCT, there is no added risk based on this measure alone.
CH measures the cornea’s ability to absorb and dissipate energy. (Hysteresis is defined as the difference between the pressure at which the cornea bends inward during an airjet applanation and the pressure at which it bends out again.) A low CH value correlates with a greater risk of glaucoma progression. It is associated with changes in the optic nerve, studies suggest.4,5 The Ocular Response Analyzer (Reichert) is the only tonometer that measures CH.
VISUAL FUNCTION
Measuring visual function is essential when monitoring glaucoma disease progression, as loss of sensitivity to light is a hallmark of progressive disease.6
Tools to aid in evaluation. Perimeter and pattern electroretinography (PERG).
VF testing via perimetry is a valuable aid in assessing optic nerve function, as it measures central and peripheral vision, both of which are negatively affected by glaucoma. This test allows for many options, including 30-2, 24-2, 10-2, Esterman binocular and short-wavelength automated perimetry. VF testing for glaucoma has relied on testing from the 30-2 and 24-2 patterned tests, as most of the nerve ganglion cells are located within the central 30 degrees of fixation.7 Recent studies show the need for the central 10-2, even in early disease, and alternating is recommended for some patients to gather the fullest picture of VF damage.8
PERG is a new method for assessing the retinal ganglion cell layer. The pattern electroretinography is recorded in response to a reversing black and white checkerboard as peaks and troughs. The Pattern Electroretinogram for Glaucoma Detection (PERGLA) is a novel approach to utilizing PERG in glaucoma. It could allow for the earlier detection of glaucoma, as found in a recent study, which shows that PERG signals could anticipate an equivalent loss of retinal nerve fiber layer (RNFL) as seen on OCT by a mean of eight years.9
OPTIC NERVE EVALUATION
Because the optic nerve fiber layer undergoes irreversible damage as glaucoma progresses, it’s important to assess optic nerve health in glaucoma patients to determine the best course of action treatment-wise.
Tools to aid in evaluation. Stereo biomicroscopy, perimetry (explained above), a fundus camera, SD-OCT and OCTA.
The handheld lenses used for stereo biomicroscopy can influence the grading. It is recommended to evaluate the optic nerve with a 78D, 60D or super 66D lens, assessing the color, placement (tilt), signs of peripapillary atrophy, vertical height, presence or absence of Drance hemorrhages, cupping, symmetry and thickness of the NFL. The red-free filter will enhance the view of loss of the NFL, and practice will allow the observer to appreciate subtle defects, such as slit defects, and more obvious defects, such as wedge defects.
Available in multiple forms, such as handheld and desktop, a fundus camera allows for imaging of the optic nerve head. The stereo disc photography options enable the provider to review images through time for subtle changes to the NFL. NFL dropout with slit defects can often be detected, as well as Drance hemorrhages — both of which can often be overlooked during dilated view of the optic disc.
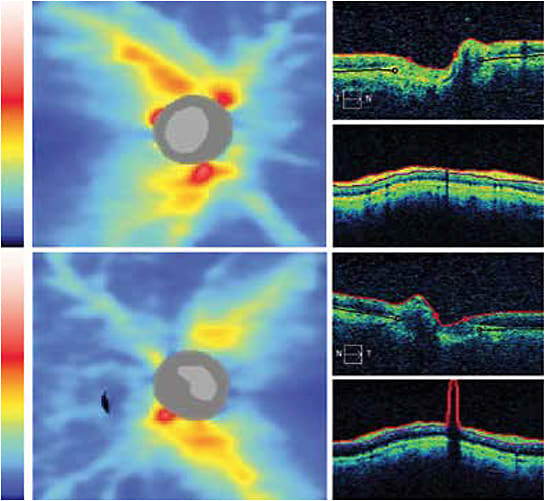
SD-OCT, or Spectral Domain-OCT, enables the analysis of the RNFL and the ganglion cell layer and comparison through time. For some patients, such as those with cognitive or physical impairments, OCT is the most reliable test because of its objective nature. OCT is not without its limitations; macular disease limits the ganglion cell analysis, and one must be astute to “red” disease.
OCTA, or OCT angiography, allows for the evaluation of the microcirculation of the optic nerve and peripapillary retina. OCTA shows a reduction in retinal and optic nerve head vessel densities and blood flow indexes in glaucoma patients, reports many studies. It’s unclear whether OCTA provides additional detection and monitoring information.10
RISK CALCULATOR
As a result of the OHTS, the Washington University School of Medicine, in St. Louis, put together a Risk Calculator to evaluate the progression of ocular hypertension on primary open angle glaucoma. It is available at https://ohts.wustl.edu/risk/ .
ANGLE BETWEEN IRIS AND CORNEA
Many of the treatments we offer to patients focus on the trabecular meshwork: medications focusing on outflow, surgical procedures, such as SLT, MIGS, trabeculectomy and valves.
Tools to aid in evaluation. Gonioscopy and OCT.
Gonioscopy allows a three or four-mirror view of the angle, enabling the practitioner to assess the ocular anatomy, specifically determining whether there is an angle closure mechanism, evaluating any trauma/dysgenesis or other abnormalities of the angle. Choose a lens and a grading scale (Spaeth, Shaffer or Scheie) you are comfortable with. (I recommend practicing gonioscopy on all patients to view normal and abnormal angles and to make the comparison easier.)
OCT anterior imaging documents angle structures. These photos also can be helpful in educating the patients about their disease and the importance of complying to their prescribed treatment(s), surgery, medication, etc.
BLOOD PRESSURE
In-office monitoring of blood pressure (BP) allows for the calculation of the patient’s ocular perfusion pressure (OPP). Mean OPP = 2/3 DBP + 1/3(SBP – DBP) – IOP. Lower perfusion pressure is strongly associated with an increased prevalence of POAG.11 It is an important diagnostic measure for patients who have disease progression, despite adequate IOP control. In addition, BP measurements are useful to gain in-office before and after prescribing a beta blocker or alpha adrenergic agonist.
Tool to aid in evaluation. An automated oscillation sphygmomanometer. This new technology increases the objectivity and repeatability of BP measurements and is easy for a technician to use.
WHAT’S NEXT
A smart silicone contact lens with an embedded micro-electromechanical system and a thin microfabricated platinum titanium strain gauge that measures the changes in corneal curvature is available for 24-hour IOP recording. Measurements are taken every five minutes for a duration of 30 seconds, giving a total of 288 measurements through a 24-hour period.12 The device, called Triggerfish (Sensimed), received FDA approval for marketing in 2016.
In addition, studies show that increased trans-lamina cribrosa pressure difference, or the difference in IOP and orbital cerebrospinal fluid pressure, may be a predictor of glaucoma. An app, developed in 2005, called STAR (Scoring Tool for Assessing Risk), can provide this assessment for glaucoma. Perhaps, this diagnostic tool will be at our disposal in the future to better serve our patients? OM
REFERENCES
- Chang DS, Arora KS, Boland MV, Supakontanasan W, Friedman DS. Development and Validation of an Associative Model for the Detection of Glaucoma Using Pupillography. American Journal of Ophthalmology. 2013;156(6):1285-1296. doi:10.1016/j.ajo.2013.07.026.
- Sample PA, Weinreb RN, Boynton, RM. Acquired dyschromatopsia in glaucoma. Survey of Ophthalmology, 31:1, 54 - 64. doi: 10.1016/0039-6257(86)90051-2
- Kersey T, Clement CI, Bloom P, Cordeiro MF. New trends in glaucoma risk, diagnosis & management. The Indian Journal of Medical Research. 2013;137(4):659-668.
- De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. Journal of Glaucoma. 2012;21(4):209-213
- Vu DM, Silva FQ, Haseltine SJ, Ehrlich JR, Radcliffe NM. Relationship between corneal hysteresis and optic nerve parameters measured with spectral domain optical coherence tomography. Graefe’s Archive Clinical and Experimental Ophthalmology. 2013;251(7):1777-1783
- Jampel HD, Signh K, Lin SC, et al. Assessment of Visual Function in Glaucoma. Ophthalmology. 118(5):986–1002
- Hebel R, Hollander H. Size and distribution of ganglion cells in the human retina. Anatomy and Embryology. 1983;168(1):125-136.
- Asaoka R. Mapping glaucoma patients’ 30-2 and 10-2 visual fields reveals clusters of test points damaged in the 10-2 grid that are not sampled in the sparse 30-2 grid. PLOS ONE. 2014;9(6):e98525. doi: 10.1371/journal.pone.0098525
- Banitt MR, Ventura LM, Feuer WJ, et al. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Investigative Ophthalmology & Vision Science. 2013;54(3):2346-2352.
- Wan KH, Leung CK. Optical coherence tomography angiography in glaucoma: a mini-review. F1000Research. 2017;6:1686. doi: 10.12688/f1000research.11691.1. eCollection 2017.
- Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Archives of Ophthalmology. 1995;113(2):216–221.
- Mansouri K, Weinreb RN. Continuous 24-hour intraocular pressure patterns monitoring for glaucoma with a contact lens sensor - time for a paradigm change. Swiss Medical Weekly. 2012;142:w13545.




