The statistics are undeniable — diabetes is a major public health problem. Nearly half of all Americans are affected. More than 30 million U.S. adults have the disease (7 million of them are unaware they have it) and 84 million have prediabetes.1 If current trends continue, the prevalence of diabetes will have increased by 54% to more than 54.9 million Americans between 2015 and 2030.2
Diabetic retinopathy and its associated pathology, including diabetic macular edema (DME), is the leading cause of vision impairment and blindness in Americans of working age (20 to 74 years).3 Given the predicted increase in diabetes, it is expected that diabetic retinopathy will also be on the rise. In fact, the National Eye Institute projects diabetic retinopathy to climb to 11 million by 2030.3
Reducing vision-threatening diabetes complications requires efforts on many fronts, so much so that the American Diabetes Association (ADA) recently published a new position statement on diabetic retinopathy, the first such update since 2002.4
This article addresses some best practices for referral of patients with diabetes, including patient education, early diagnosis, diagnostic technologies, treatment, telemedicine screening, and collaborative care among members of the healthcare team to produce the best outcomes.
Promote Health Literacy
A major hurdle in diabetes care is poor health literacy, which is prevalent among individuals with diabetes and has been associated with increased diabetes-related complications. Eyecare professionals tend to see diabetes patients more often than their primary care providers do, therefore, we play an important role in counseling patients on modifiable risk factors, the ABCs of diabetes — (A) glycosylated hemoglobin (HbA1c), (B) blood pressure, (C) cholesterol, and (S) smoking cessation — to reduce the risk or slow the progression of diabetic retinopathy. The patient described in case 1 — a 52-year-old black female with an HbA1c of 11 and proliferative diabetic retinopathy (PDR) in both eyes — is an example of someone who would benefit from such counseling.
REMOTE RETINAL SCREENING
Multiple studies have argued both in support of and against the idea that telemedicine is an improvement over eye care provider-based screening.1,2 The recently published ADA position statement discussed retinal telemedicine screening for diabetic retinopathy. Although there is no consensus, this may be an effective means of identifying diabetic retinopathy in people living in underserved areas, perhaps where the providers-to-patients ratio is low or where the distance to reach a provider is prohibitive, particularly when the alternative is no screening.
Retinal photographs are not a substitute for comprehensive dilated eye examinations, but they may alert providers to the presence of disease and open a dialogue with patients about the need for prompt treatment and regular follow-up.
References
- Phan AD, Koczman JJ, Yung CW, Pernic AA, Doerr ED, Kaehr MM. Cost analysis of teleretinal screening for diabetic retinopathy in a county hospital population. Diabetes Care. 2014;37(12):e252-253.
- Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120(12):2604-2610.
Good glycemic control (HbA1c ≤7), as observed in major diabetes studies, is key to reducing or preventing progression of diabetic retinopathy.5-7 Yet, a study evaluating perceptions of diabetes control found that a high proportion of patients believe they have “good” or “excellent” control of their diabetes, despite an average HbA1c of 9.5.8 We recommend asking patients about their HbA1c in simple terms that they understand, such as “your 3-month blood sugar” results, and emphasizing the direct link between these readings and disease progression.
According to recently published hypertension guidelines, patients with diabetes should make sure their blood pressure is 130/80 mm Hg.9 As observed in the Fenofibrate Intervention and Event (FIELD) study, cholesterol-lowering medications are beneficial in slowing the progression of diabetic retinopathy.10 Advise patients to stop smoking, as it can exacerbate vascular disease, and emphasize proper nutrition and weight loss for those with diabetic retinopathy.
Case 1: 54-year-old black female with HbA1c of 11 and proliferative diabetic retinopathy in both eyes
We also encourage patients to record their ABCs along with their current medications in a journal that they bring to every appointment. Not only does this useful tool encourage patients to feel ownership over their disease, it also facilitates communication with their healthcare professionals.
Other measures include providing educational information and brochures to patients in their preferred language and in large print. Trained staff members also can assist in diabetes education.


Stress the Need for Regular Dilated Examinations
To detect signs of sight-threatening retinopathy, all patients with diabetes should have dilated retinal exams early and regularly to identify problems and ensure that treatment begins promptly. Yet, too many patients do not show up for regular examinations and evaluations. According to a recent study, about 60% of Americans with diabetes do not adhere to recommendations for annual eye examinations.11 Therefore, all members of the diabetes health care team must consistently reinforce the importance of regular dilated retinal examinations. (See “Remote Retinal Screening,” page 17.)
All patients with type 2 diabetes should receive annual dilated retinal examinations beginning at diagnosis. Patients with type 1 diabetes should receive a dilated retinal examination within 5 years of disease onset, and annually thereafter. All women with diabetes who become pregnant should have a dilated retinal examination during each trimester of pregnancy.
Although longer disease duration is an important predictor of diabetic retinopathy, about 30% of patients with type 2 diabetes have diabetic retinopathy at the time of diabetes diagnosis.12 Most of these patients likely had diabetes for several years before they were diagnosed. Case 2 is an example: a 48-year-old Hispanic male without a history of diabetes who has diabetic retinopathy.
With these realities in mind, we must ensure patients schedule follow-up appointments before they leave our offices, and we must have a reliable reminder system, which may include text messages and phone calls, to prompt patients to attend their appointments.
Case 2: 48-year-old Hispanic male with moderate diabetic retinopathy at the time of diabetes diagnosis
Stay Current With Imaging Technology
Recent advances in imaging technologies have significantly improved our ability to detect diabetic retinopathy and maculopathy. Although baseline retinal photography is still considered the gold standard for diabetic retinopathy imaging, it has some drawbacks that limit its use in clinical work. For instance, standard retinal photographs limit the view to about 30 degrees of the posterior pole and can sometimes miss early signs of diabetic retinopathy. The introduction of ultra-widefield (UWF) imaging has changed the landscape.
UWF imaging allows for a larger field of view, so we can see more of the retina and detect peripheral changes. This is illustrated by case 3, a patient who had diabetic retinopathy lesions in the periphery. Not only does this technology facilitate early detection, but it also gives us a platform for educating patients on the importance of follow-up care.


Case 3: Ultra-widefield imaging detected peripheral diabetic retinopathy lesions in a 60-year-old white male with proliferative diabetic retinopathy
In fact, UWF imaging is becoming the new standard for detecting diabetic retinopathy. Various studies have found that peripheral lesions suggest more severe disease in about 10% of eyes.13 (See “Diabetic Retinopathy Severity Scale, page 22.) The study also evaluated the effect of these peripheral lesions on retinopathy progression and found that eyes with peripheral lesions had a 2.2-fold increased risk of progressing from mild to possibly moderate disease. For some patients, the study found a 3.2-fold increase in risk of progressing from mild to severe disease.13
OCT and OCT angiography (OCTA) have dramatically improved early detection and care of diabetic retinopathy and maculopathy. OCT allows for the early identification and management of DME, rather than the presence of clinically significant macular edema, a diagnosis made by macula slit lamp examination. Currently, DME is categorized as center-involved versus non-center-involved based on spectral domain OCT. Center-involved DME is characterized by loss of foveal contour, cystoid macula edema (CME) involving the center of the fovea, neurosensory detachment involving the center of the fovea and increased central subfield thickness as shown in case 4. Non-center-involved DME is characterized by retinal thickening and/or cystic spaces not directly involving the center of the macula.

Case 4: 57-year-old male with center-involved DME of the left eye; note improvement status post anti-VEGF therapy
OCTA detects blood flow without the use of intravenous dye, therefore eliminating the risk for complications, such as anaphylaxis. It is an excellent tool to detect subclinical microaneurysms, the earliest sign associated with diabetic retinopathy, that are often not perceived through a dilated retinal examination as depicted in case 5.



Case 5: OCTA imaging detected multiple hyper-reflective microaneurysms and neovascular changes of PDR
OCTA can detect other vascular anomalies, such as vascular loops, tortuosity, and dilation of the vessels, as well as intraretinal microvascular abnormalities and superficial neovascularization. It also detects diabetic macular ischemia (DMI) with clinical signs of paramacular areas of capillary nonperfusion, impairment of the choriocapillaris flow, and enlargement of the foveal avascular zone (FAZ). Abnormalities in the structure or perfusion of the FAZ not only results in vision impairment but a poor prognosis, because the condition cannot be treated. DMI should be ruled out in patients with poor vision at presentation or despite attempted treatment for DME.
All patients with diabetic retinopathy should be monitored closely with follow-up examinations every 3 months. However, patients with severe nonproliferative diabetic retinopathy (NPDR), PDR, and DME should be referred to a retina specialist, even patients with 20/20 vision and no visual complaint as seen in case 6.


Case 6: Asymptomatic PDR patient with an area of neovascularization observed on UWF angiography
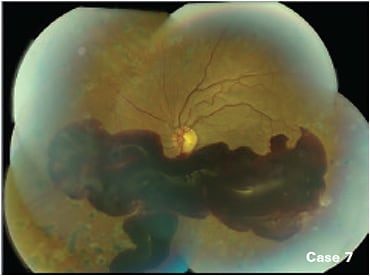
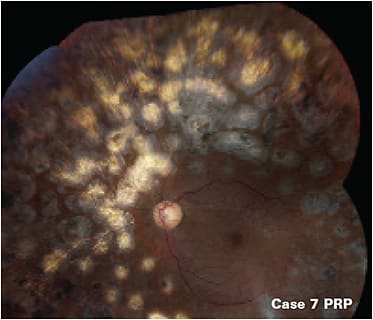
Anti-VEGF is the first-line treatment for any patient with center-involved DME and PDR.14,15 In some cases of persistent edema after three to six injections, the retina specialist may elect to switch to a different anti-VEGF agent, add laser, or use a steroid. For patients with non-center-involved DME, treatment may be focal laser, anti-VEGF, or observation if vision is not compromised. However, some cases, such as case 7 benefit from PRP.


Case 7: Non-clearing vitreous hemorrhage treated with PRP
Collaborative Care
It is important to establish partnerships with other health care providers and provide consistent communication with all providers who participate in the care of patients with diabetes. A progress report should be sent in a timely manner to the patient’s primary care physician or health care team, even when no diabetic retinopathy is detected. This is an important component of HEDIS (the Healthcare Effectiveness Data and Information Set), as primary care physicians are required to obtain documentation that annual eye examinations were performed on their patients with diabetes.
When coordinating care with a retina specialist, it may not be ideal to rely on the patient to schedule appointments, but rather the referring doctor should make an appointment for the patient. Primary care providers should also be informed if additional care is warranted, such as referrals to a retina specialist. An exam summary report should be given to the patient and faxed to the retina specialist.
To ensure that patients adhere to the recommended care, a follow-up appointment should be made to the primary eye care physician’s office. This ensures continuity of care and allows patients to discuss any concerns they may have about treatment.
Rein in Diabetes
Battling the emerging epidemic of diabetic retinopathy requires collaboration by all members of the diabetes health care team to ensure better outcomes for these patients. The new ADA position statement not only provides valuable clinical practice updates and recommendations regarding diabetic retinopathy, it also may serve as a guide to improve interaction among the patient’s entire health care team to prevent the onset and progression of diabetes-related vision loss. ■
DIABETIC RETINOPATHY SEVERITY SCALE
In an effort to improve communication between eye care providers and primary care physicians caring for patients with diabetes, the latest diabetic retinopathy scale is provided here.1
- Diabetic retinopathy absent: no abnormalities
- Mild nonproliferative diabetic retinopathy (NPDR): microaneurysms only
- Moderate NPDR:
–More than just microaneurysms but less than severe NPDR
–Intraretinal microaneurysms and dot and blot hemorrhages of greater severity, in 1 to 3 quadrants
–Cotton wool spots, exudates, venous caliber changes, including venous beading, and intraretinal microvascular abnormalities are present but mild1 - Severe NPDR:
–Any of the Early Treatment Diabetic Retinopathy Study (ETDRS) 4-2-1 criteria
–The ETDRS “4-2-1 rule” indicates the presence of severe intraretinal hemorrhages (>20) and microaneurysms in each of 4 quadrants, venous beading in ≥2 quadrants, or intraretinal microvascular abnormality in ≥1 quadrants
–No signs of proliferative diabetic retinopathy - PDR: ≥1 of the following:
–Neovascularization
–Vitreous/preretinal hemorrhage
Reference
- Wilkinson CP, Ferris FL III, Klein RE, et al; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677-1682.
References
- Diabetes Report Card 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2018.
- Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6-12.
- National Eye Institute. Diabetic Eye Disease Projected To Increase Among U.S. Population. 2014. Available at: https://www.nei.nih.gov/sites/default/files/nehep-pdfs/GM_DED_drop-in%20article_2014.pdf ; last accessed April 23, 2019.
- Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412-418.
- Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99-111.
- Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
- Gopalan A, Moss H, Tao Y, Zhu J, Volpp K. Patient perceptions of current disease control in poorly controlled diabetes. Health. 2014;6(15):1964-1971.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324.
- Keech AC, Mitchell P, Summanen PA, et al.; FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687-1697.
- Murchison AP, Hark L, Pizzi LT, et al. Non-adherence to eye care in people with diabetes. BMJ Open Diabetes Res Care. 2017;5:e000333.
- Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815-819.
- Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-2595.
- Nguyen QD, Brown DM, Marcus DM, et al.; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801.
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146.



