The introduction of OCT as in vivo histology nearly 20 years ago revolutionized the clinical evaluation of the ocular fundus. Initially utilizing time-domain algorithms, OCT produced 2D cross-sectional representations of the retina based on low coherence interferometry and reflected tissue scattering.1 Rapid advancements in the technology resulted in the introduction of Fourier- or spectral-domain OCT, which is high-resolution scanning that has allowed superb correlation with clinical findings. (Colored fundus photography (CFP) and OCT images seen in Figure 1.)

Using patient examples, this article will illustrate how the capabilities of contemporary OCT have aided optometrists in managing glaucoma, macular diseases, pigmented and elevated lesions and elevated optic discs.
GLAUCOMA
Imaging for suspected glaucoma has focused recently on the inner retina that is dense with ganglion cells. This is significant, as early structural changes appear to be the initial deficit of the disease and, in terms of making the diagnosis, superior to retinal nerve fiber layer (RNFL) thickness parameters.2,3 An example of a patient with such early changes in macular thickness follows:
A 55-year-old male was referred from a community screening as a glaucoma suspect. While his ocular history was non-contributory, he carried a diagnosis of sleep apnea with CPAP use, a potential risk factor for glaucoma. There was a questionable vessel deflection inferiorly in his right eye compared with similar vasculature in the left eye. VFs were unremarkable OU. OCT analysis, however, revealed corresponding statistically significant RNFL thinning, and the device’s ganglion-cell map showed minimal ganglion-cell thinning that respected the horizontal raphe and was repeatable. (See Figure 2) Given these baseline data and following a detailed discussion with the patient, he was given a 6-month follow-up.
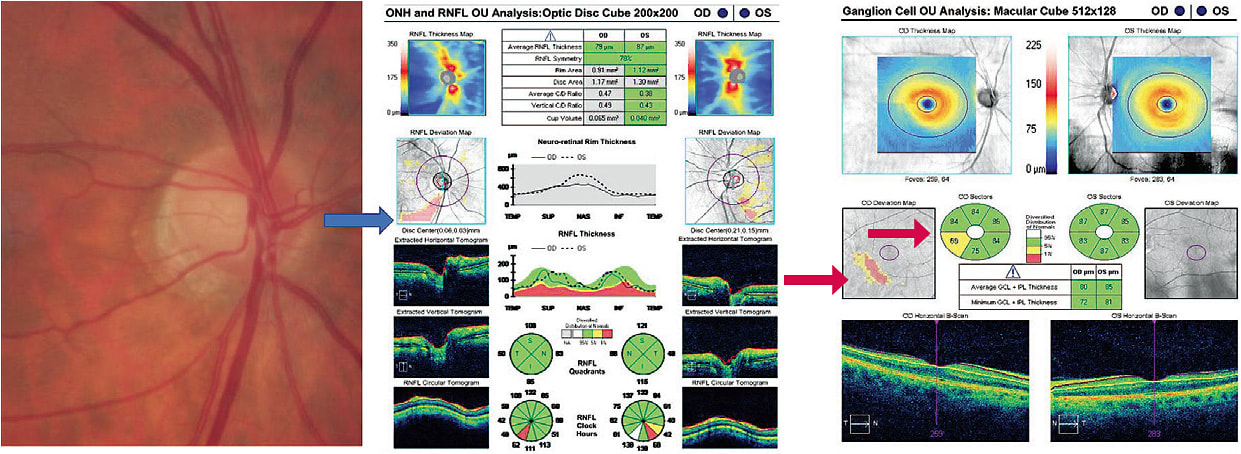
The next example illustrates stability of RNFL thickness in a 19-year-old myopic patient who was followed as a glaucoma suspect, due to his large optic discs and C/D ratio. His VFs had been clear over the several years of observation. The proprietary quantitative data support stability. (See Figure 3.) The right and left eye data are similar, so the right eye is shown for sake of brevity. The follow-up period is reassuring for stability and a recommendation for regular periodic visits.

MACULAR DISEASES
Turning our attention to the role of OCT in macular diseases, the first example involves a full-thickness macular hole in a patient who had been followed for several months. At her last appointment, this 55-year-old female was deemed a surgical candidate. She underwent vitrectomy that included membrane release. (See Figure 4.) Interestingly, the post-operative OCT showed the re-establishment of the external limiting membrane. This crucial observation established the diagnosis of diabetic macular edema and applies in post-operative cases following FTMH, as well as wet AMD.4 On follow-up, the patient had an immediate post-operative VA of 20/200, but recovered to 20/40 with re-population of the photoreceptor layer over the course of nine months.
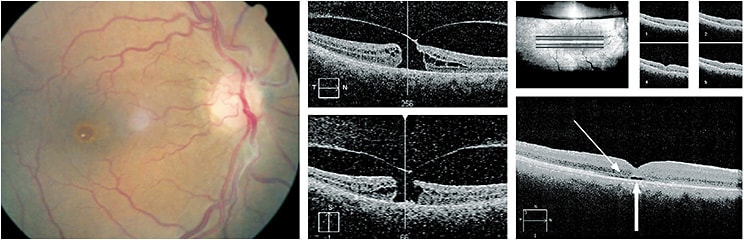
The previous example shows the relationship between the detached vitreous with remaining traction (causing the FTMH). The patient had vitreo-macular adherence in the fellow eye, a condition with a far better prognosis. The key post-operative observation here is of an intact/re-established external limiting membrane.
Characterization of the relationship between the vitreous and macula has been aided significantly by cross-sectional imaging of that area and volumetric scanning, which allows 3D visualization. For example, a 58-year-old female presented with a two-week history of reduced vision in her right eye. Her history was non-contributory. VA was 20/60 in the involved eye. Figure 5 (p.20) shows a subtle difference in appearance between eyes, but a distinct difference in the cross-sectional scans. The distinction between these presentations is that of vitreo-macular traction right eye and adhesion left eye. Traction disrupts macular anatomy, while adhesion leaves macular anatomy intact.5,6 In this case, the risk of macular hole is present and, therefore, warrants close observation over the next three months, as opposed to consideration for intravitreal ocriplasmin, for example, for the right eye. The left eye would do well with regular periodic ophthalmic surveillance.7 The application of OCT in post-operative analysis has led to a comprehensive guide for pharmacological interventions.8

PIGMENTED AND ELEVATED LESIONS
Other applications of OCT include the imaging of pigmented and elevated lesions of the ocular fundus. An example: a 57-year-old female was referred for imaging of an elevated retinal lesion, which had been observed without change over several years. Baseline clinical characteristics did not qualify it as high risk.9,10 The OCT allowed for baseline quantification of lesion height. In addition, an incidental, but clinically invisible, retinal pigment-epithelial detachment was seen (Figure 6).

APPLICATIONS ABOUND
OCT has a number of applications in primary ophthalmic practice. Examples include, but are not limited to, early diagnosis of glaucoma, macular diseases, as well as conditions associated with abnormal vitreous attachment and suspicious elevated retinal lesions and the dilemma often accompanying elevated optic discs, as illustrated above. OM
References
1. Huang D, Swanson EA, Lin CP, et al. Optical Coherence Tomography Science. 1991 Nov 22;254(5035):1178-81.
2. Dascalescu D, Corbu C, Coviltir V, et al. The ganglion cell complex as an useful tool in glaucoma assessment. Rom J Ophthalmol. 2018 Oct-Dec;62(4):300-303.
3. Chen MJ, Yang HY, Chang YF Hsu CC, Ko YC, Liu CJ. Diagnostic ability of macular ganglion cell asymmetry in Preperimetric Glaucoma. BMC Ophthalmol. 2019 Jan 8;19(1):12. doi: 10.1186/s12886-018-1019-4.
4. Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. Am J Ophthalmol. 2010 Jul;150(1):63-67.e1. doi: 10.1016/j.ajo.2010.01.039. Epub 2010 May 10.The Association Between Percent Disruption of the Photoreceptor IS–OS and Visual Acuity in Diabetic Macular Edema.
5. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013 Dec;120(12):2611-2619. doi: 10.1016/j.ophtha.2013.07.042. Epub 2013 Sep 17.
6. Rodman JA, Shechtman D, Sutton BM, Pizzimenti JJ, Bittner AK; VAST Study Group. Prevalence of Vitreomacular Adhesion in Patients Without Maculopathy Older Than 40 Years. Retina. 2018 Oct;38(10):2056-2063. doi: 10.1097/IAE.0000000000001792.
7. Stalmans P, Delaey C, de Smet MD, van Dijkman E, Pakola S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial). Retina. 2010 Jul-Aug;30(7):1122-7. doi: 10.1097/IAE.0b013e3181e0970a.
8. Stalmans P, Duker JS, Kaiser PK, et al. Oct-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina. 2013 Nov-Dec;33(10):2003-11. doi: 10.1097/IAE.0b013e3182993ef8. Review.
9. Shields CL1, Dalvin LA, Ancona-Lezama D, et al. Choroidal Nevus Imaging Features in 3,806 Cases and Risk Factors for Transformation into Melanoma in 2,355 Cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2018 Dec 31. doi: 10.1097/IAE.0000000000002440.
10. Shields CL, Demirci H, Materin MA, Marr BP, Mashayekhi A, Shields JA. Clinical factors in the identification of small choroidal melanoma. Can J Ophthalmol. 2004 Jun;39(4):351-7. Review.




