The population continues to age, bringing with it more ocular disease and, therefore, more of a need for us to care for patients who have glaucoma. Disappointingly, 20% of optometrists write 80% of glaucoma prescriptions, according to Allergan. Although we have no data as to why this is, I wonder whether it could be the presumed complexity of getting started in diagnosing glaucoma. The good news: Now, more than ever, an array of diagnostic tools, when used in concert, can facilitate the diagnosis of glaucoma.*
Here, I discuss these diagnostic tools, listed in alphabetical order, and provide associated clinical pearls.
DIAGNOSIS HOW TO
→ IN PATIENTS LABELED AS “GLAUCOMA SUSPECTS,” there are often no smoking guns, making systematic diagnostic testing necessary to help O.D.s confirm their clinical suspicions. Below is a sample flow chart of a typical glaucoma diagnostic pathway.
VISIT 1: Initial comprehensive examination with funduscopic suspicion of glaucoma seen stereoscopically through the biomicroscope. Review vascular risk factors.
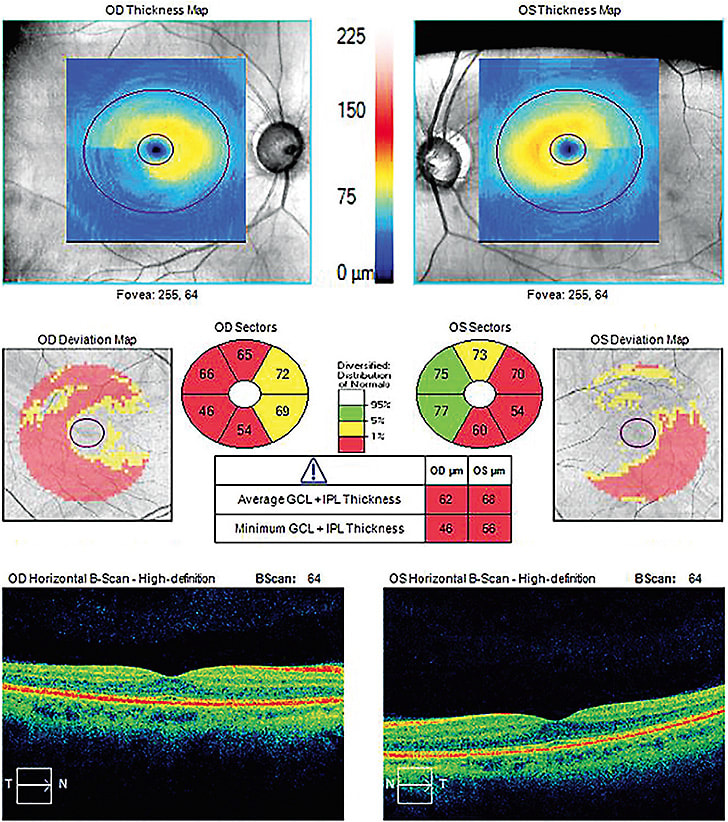
VISIT 2: Tonometry, gonioscopy, pachymetry, hysteresis, structural testing (RNFL and GCC on OCT, Vessel Density on OCT-A)
VISIT 3: Functional testing (perimetry, ERG), tonometry
VISIT 4: Repeat functional testing, tonometry
VISIT 5: Repeat structural testing, tonometry
CORNEAL HYSTERESIS (CH) MEASUREMENT DEVICE
CH is the cornea’s capacity to absorb and release energy when applanated. Given that glaucoma involves the biomechanical properties of the optic nerve and that the cornea, sclera, peripapillary ring and lamina cribrosa are essentially derived from the same extracellular matrix, it has been proposed that measuring CH may actually represent optic nerve head (ONH) biomechanics, reports Current Opinion in Ophthalmology. CH is part of the optometrist’s overall comprehensive glaucoma risk profile.
Clinical pearl: We should note that the CH value of a healthy patient is roughly 10.5 mmHg, according to Ophthalmology. Thus, patients who have an 10.5 mmHg value or higher have a ONH that is able to resist deformation from the many forces it is exposed to.
Conversely, a CH value of below 10.5 mmHg would indicate a reduced capacity to do so, increasing the likelihood of glaucomatous damage. Something else to keep in mind: CH and central corneal thickness (p.23) are “highly correlated and associated with VF progression,” reports Journal of Glaucoma.
GONIOSCOPY
This is critical for determining glaucoma type and, thus, management. Specifically, gonioscopy establishes whether the angle is open, provides qualitative data about the angle, such as the presence or absence of synechiae, abnormal vessel growth and recession from previous trauma, and it allows for the dynamic assessment of the interaction between the peripheral iris and trabecular meshwork.
Clinical pearl: Undoubtedly, gonioscopy takes practice, but it is a necessary skill to master when electing to manage glaucoma. This is because it allows the O.D. to understand the mechanism of the disease and, thus, how it may play out. If O.D.s can’t acquire this information, they may not be aggressive enough in their management. (A well-known and excellent resource for improving gonioscopy is www.gonioscopy.org .)
PACHYMETER
Pachymeters measure central corneal thickness (CCT). This is important because, on average, thin CCT may result in underestimated IOP, and thick CCT may result in an overestimation of IOP via tonometry. More importantly, CCT is an independent risk factor for the development of glaucoma.
Clinical pearl: Optometrists should note that an average CCT below 555 μm, is considered thin and places a patient at higher risk for glaucoma, reports the Ocular Hypertension Treatment Study (OHTS). Conversely, a CCT over 588 μm is considered thick, tending to confer a protective effect.
PERIMETER
This quantitatively assesses the function of retinal GCC at various retinal locations, 54 such loci on 24-2 testing and 68 on 10-2 testing, known susceptible to damage. A patient could have thick neuroretinal tissue, but damaged retinal GCC. Conversely, a patient could have thin neuroretinal tissue, but the retinal ganglion cells are functioning fine. Perimetry reveals VF defects, via deviation maps, on a printout.
Clinical pearl: Optometrists should analyze the data provided by perimetry to look for known patterns of loss, such as nasal step, arcuate and paracentral defects, consistent with glaucoma, in the process of ruling out non-glaucomatous defects. Aligning clinical observation with glaucomatous data from perimetry helps to ensure diagnostic accuracy.
Additionally, perimetry should be used to monitor for progressive retinal ganglion cell damage. The World Glaucoma Association recommends at least two VFs in the first six months of treatment, followed by at least two additional VFs in the subsequent 18 months to aid in detecting the progression of the disease. However, test frequency should always be customized to the individual’s disease state. (Notably, there is often confusion that occurs when structural and functional testing do not perfectly correlate. This relationship is complicated by how respective technologies acquire and display their data. As a result, perfect correlation between structure and function is the exception to the rule and should not discourage us from feeling confident in our diagnoses.)
It should be noted that pattern electroretinography (PERG) and the photopic negative response of full-field ERG, also known as PhNR, provide an additional, minimally invasive and objective tool for assessing retinal ganglion cell function. (For additional information, visit https://bit.ly/2Ee5odg .)
PUPILLOMETER
Glaucoma is typically an asymmetric disease. The variability and lack of precision with conventional pupillary testing by the swinging flashlight test hampers its ability to aid in quantifying this asymmetry and, thus, its clinical utility. Automated infrared pupillometers objectively and repeatably quantify abnormal pupil responses and can be used adjunctively to confirm the extent of asymmetric ONH damage in patients who have glaucoma. A study in the Indian Journal of Ophthalmology shows that there is good correlation between the magnitude of relative afferent pupillary defects (RAPD) and cup-to-disc ratio, mean deviation of VF testing and RNFL layer measurements. This suggests pupillometry may be beneficial as a screening tool for asymmetric glaucoma.
Clinical pearl: Objective measures of RAPD with an infrared pupillometer yields high sensitivity and specificity in the detection of primary open-angle glaucoma. This assessment, combined with ONH evaluation, OCT evaluation of RNFL and GCC layer thickness, as well as the mean deviation on VF testing will improve diagnosis and management of our glaucoma patients, notes Leslie O’Dell, O.D., who recently launched the Facebook group “Glaucoma Docs.” (See: bit.ly/FBFGlaucomaDocs .)

RETINAL PHOTOGRAPHY
Photography of the ONH and surrounding RNFL provides a qualitative overview of the neuroretinal tissue that can be affected by glaucoma, as well as a historical record from which to compare. Additionally, the OHTS shows 84% of disc hemorrhages not seen clinically were detected with the aid of retinal photography.
Clinical pearl: In my practice, we mandate that photographs focusing on the neuroretinal rim and lamina cribrosa be taken separately, as this practice enhances our assessment by capturing both the neural and connective tissue that is damaged in glaucoma. (To improve glaucoma evaluations, optometrists should check out the Glaucomatous Optic Neuropathy Evaluation [GONE] project as a resource: https://bit.ly/34jI89d .)
SCANNING TOMOGRAPHY
SD-OCT, OCT-A and OCT fall under here. To start, SD-OCT provides measurements of the thickness of the neuroretinal tissue that is characteristically damaged in glaucoma; the RNFL, the GCC and the neuroretinal rim. Specifically, various OCT platforms present neuroretinal tissue thickness data in comparison to their respective reference databases of healthy patients, displaying the tissue as normal, borderline or outside normal limits.
OCT-A employs motion contrast (comparison of sequential B scans of the same static retinal or ONH area) to detect the movement of red blood cells. This allows for a non-invasive, quantitative assessment of the vascular health.
Clinical pearl: Optometrists should look to OCT-A as a means of demonstrating the reduced density of vasculature at the optic nerve in the circumpapillary retina and maculas of patients who have early glaucoma.
OCT enables the early diagnosis of glaucoma. In fact, a study in Ophthalmology shows glaucomatous RNFL were detected up to eight years prior to detection of damage with standard perimetry.
Clinical pearl: O.D.s should be aware that thin tissue is likely indicative of worse disease, and thick tissue is more likely indicative of good tissue health. Additionally, to first confirm that the tissue thinning is consistent with glaucoma, O.D.s should analyze the macular and ONH data together. After all, failure to look at all tissues that can be affected can result in failure to accurately diagnose the extent of glaucomatous damage. (For additional information on this technology, see https://bit.ly/38ABFtc .)
TONOMETER
This device estimates IOP, the only modifiable glaucoma risk factor. It applies force to the cornea. In general, the higher the estimated IOP, the higher the risk of glaucoma, though it is important to note that this is not absolute.
Clinical pearl: Optometrists should keep in mind that the cause of glaucomatous optic neuropathy is multifactorial, with the optic nerve exposed to forces beyond IOP, most notably ocular perfusion pressure and cerebrospinal fluid pressure. (See https://bit.ly/2LN5jBJ for additional information.)
Additionally, one tonometry reading should be viewed as a brief snapshot of a patient’s IOP, as IOP fluctuates constantly throughout the day and night. Therefore, O.D.s should obtain multiple tonometry readings during different times a day to get a clearer understating of IOP range and glaucoma risk.
ACHIEVING A DEFINITIVE DIAGNOSIS
Never before have there been so many tools to facilitate the glaucoma diagnosis, eliminating the presumed complexity associated with identifying the presence of the disease.
When used in concert, these diagnostic technologies aid in providing a definitive diagnosis, enabling us to institute management plans that allow our patients to maintain their vision. OM
*Fundamentally, there is no one individual diagnostic tool that aids in definitively diagnosing glaucoma, but there are rather multiple tools used in conjunction with one another, assuring diagnostic accuracy. For additional coverage on diagnostic devices that aid in identifying glaucoma, see https://bit.ly/2RQLVaW .




