Self-monitoring one’s health is becoming more mainstream. Think about the Apple Watch or Fitbit as examples. Similarly, technologies are available to aid in the monitoring of posterior segment diseases, namely AMD and glaucoma, delivering data to the health care team as adjunctive diagnostics to complement in-office visits. Given the self-quarantining and restricted in-office visits, due to the COVID-19 pandemic, it could be argued that such tools are particularly timely. Additionally, at-home monitoring could reduce the burden on the health care system without compromising patient care.
For optometrists, remote monitoring is another way we can continue to provide exceptional patient care and reduce the risk of vision loss from chronic diseases.
Here, I discuss the self-monitoring tools for posterior segment diseases.
GLAUCOMA: AT-HOME AND 24-HOUR MONITORING
With diurnal fluctuations and IOP spikes, the IOP measurements taken in the clinic may not be providing an accurate picture of the patient’s IOP throughout the day. This is where self-monitoring comes in for glaucoma patients. Two such tools are available:
- iCare Home tonometer (iCare USA). This is a fast rebound tonometer that enables self-monitoring during waking hours and does not require a topical anesthetic. The data (i.e., eye, IOP, date, time) is accessed by a health care provider via iCare Clinic software. A 2017 study shows 128/130 patients (98%) were able to use the iCare Home properly, and agreement between the device and an office-based assessment with Goldmann Applanation Tonometry was comparable.1 Also, most patients were able to perform self-tonometry, and the method was generally well-accepted.2 It is covered by Medicare. (Visit icare-usa.com/products/icare-home-tonometer/ .)

- Sensimed Triggerfish (Sensimed S.A.). This is comprised of a smart contact lens that contains a microelectromechanical system and a thin microfabricated platinum titanium strain gauge that measures IOP every five minutes for a duration of 30 seconds, providing 288 measurements through a 24-hour period.3 Clinical studies show it has high reproducibility, “suggesting that data from continuous 24-hour IOP monitoring may be useful in the management of patients with glaucoma.”4 (Visit sensimed.ch/sensimed-triggerfish .)

AMD: AMSLER GRID, MONITORING SYSTEM, APPS
Early detection of wet AMD is critical for maintaining a patient’s functional vision (20/40 or better); unfortunately many of our patients (~85%) present with a baseline VA worse than 20/40,5,6 and the average VA at the time of a first anti-VEGF injection is 20/83.
At-home monitoring could combat this. Four such tools are available:
- Amsler grid. This commonly used, home-monitoring tool has been in our arsenal for decades (available for download at amslergrid.org ) to monitor central vision and changes due to AMD or as a macular function test before cataract surgery.7 This is accomplished when patients test each eye to see whether the lines in the grid appear blurred, distorted or wavy; the boxes deviate in shape and size; the grid has dark areas or holes or the corners and sides of the grid are missing, all during fixation on the grid’s central dot. The grid re-lies upon patient compliance (which is poor); is not interactive; and there is an inherent need for good near vision to differentiate the grids, which may be difficult in patients who have other ocular comorbidities.7 Additionally, one study found that more than 60% of patients who developed wet AMD did not show Amsler grid changes.8

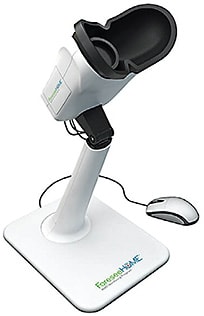
- ForeseeHome AMD Monitoring Program (Notal Vision). This tool can help detect AMD when patients who have 20/40 or better vision convert to wet AMD.9 Specifically, patients place their eyes in the device’s oculars and click on a mouse when seeing “bumps” or “waves” on a series of dotted lines. When an alert (a statistically significant change in test scores from the patient’s baseline indicating a potential wet AMD conversion) is triggered, the Notal Vision Diagnostic Clinic confirms the test data and alerts the doctor via an encrypted email. The HOME study and others reveal lesion sizes were significantly smaller at the time of detection by the tool vs. office visit detection.9-12 The key to ForeseeHome is identifying the right patient candidates: Patients should have intermediate dry AMD in one or both eyes, any large drusen (>125 μm) or pigmentary abnormalities associated with at least medium drusen (>63 μm to ≥125 μm) within two disc diameters of the fovea.13 Medicare covers the cost of both the device and the monthly monitoring service. (Visit foreseehome.com .)
- Alleye app (Oculocare). This is a smartphone- and tablet-compatible app to aid in the early detection of AMD and diabetic macular edema, as well as at-home monitoring of an existing retinal disease. Specifically, using one eye at a time, the patient places 12 points along an invisible line connecting two outer points. For patients to access the app, their eye doctor must prescribe it, and an annual license is required to connect the Alleye app to a doctor’s account. (Visit alleye.io .)
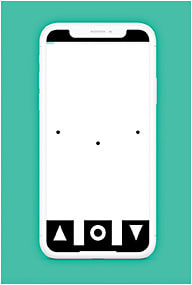
- Myvisiontrack (mVT) app (Genentech). This app is designed to identify early changes in vision in patients who have AMD and/or diabetic eye disease via having its user look at a series of shapes and tapping the shape that looks different from the others. Test data is then automatically uploaded to a physician’s portal for doctor review. Additionally, doctors are alerted to significant changes in vision. (Visit myvisiontrack.com )

TIMELY AND COMPLIMENTARY
Because the tools described above can be used by patients outside the office — ideal during the pandemic — and they provide complementary data that enables us to intervene early, it makes sense to investigate their use. OM
REFERENCES
- Takagi D, Sawada A, Yamamoto T. Evaluation of a New Rebound Self-tonometer, Icare HOME: Comparison With Goldmann Applanation Tonometer. J Glaucoma 2017;26(7): 613-618.
- Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of Intraocular Pressure by Patients With Glaucoma. JAMA Ophthalmol. 2017; 135(10):1030-1036.
- Mansouri K, Weinreb RN. Continuous 24-hour intraocular pressure patterns monitoring for glaucoma with a contact lens sensor - time for a paradigm change. Swiss Medical Weekly. 2012;142:w13545.
- Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012; 130: 1534-1539.
- Ho AC, Albini TA, Brown DM, Boyer DS, Regillo CD, Heier JS. The Potential Importance of Detection of Neovascular Age-Related Macular Degeneration When Visual Acuity Is Relatively Good. JAMA Ophthalmol. 2017;135(3):268-273.
- Rahimy E, Rayess N, Ho AC, Regillo CD. Treatment Outcomes for Neovascular Age-Related Macular Degeneration Patients with Initial Vision Better Than 20/40 Using a Treat-and-Extend Regimen. Retina. 2016;36(5):875-880.
- Tripathy K, Salini B. Amsler Grid. In: StatPearls Publishing; 2020 Jan. 2020 Jul 31.
- Loewenstein A, Malach R, Goldstein M, et al. Replacing the Amsler grid: a new method for monitoring patients with age-related macular degeneration. Ophthalmology. 2003; 110(5):966-970
- Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The Home Monitoring of the Eye (HOME) study design - HOME Study report number 1. Contemp Clin Trials. 2014;37(2):294-300.
- Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus Bevacizumab to Treat Neovascular Age-related Macular Degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399-1411.
- Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239-248.
- Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-129.
- Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851.
All images courtesy of their respective manufacturers.




