The COVID-19 pandemic has caused a vast and breakneck technological shift for a myriad of businesses, and the optometric profession and optical businesses are not immune. Specifically, more patients than ever before are foregoing spectacle and contact lens purchases in person for online purchases. As a result, now, more than ever, it is time for doctors of optometry to expand their services from refractive eye care to more medical eye care. One of the easiest ways to begin this transformation is to actively examine patients for signs of diabetic eye disease.
Here, I discuss the diagnostic criteria for diabetes and diabetic retinopathy (DR) and the diagnostic devices that can aid in diagnosis.
DIAGNOSTIC CRITERIA
To appropriately diagnose and care for these patients, we must first know the criteria of diabetes and DR, as these definitions change over time, due to researchers gaining a better understanding of this chronic disease.
The current American Diabetes Association diagnostic criteria for diabetes are:
- A1C ≥ 6.5%
- Fasting plasma glucose level ≥126 mg/dL (7.0 mmol/L)
- 2-hour plasma glucose level ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT)
- Random plasma glucose level ≥200 mg/dL (11.1 mmol/L) in a person who has classic symptoms of hyperglycemia (polyuria, polydipsia, weight loss) or hyperglycemic crisis
Separate criteria for gestational diabetes (GDM) determined through:
- The one-step two-hour 75-g OGTT, taken at 24 weeks to 28 weeks of pregnancy
- A two-step process for the screening and diagnosis of GDM.
DR is divided into two categories: nonproliferative and proliferative. Within both categories, the patient can develop diabetic macular edema (DME). The various definitions have been adapted from the Early Treatment Diabetic Retinopathy Study (ETDRS), which enumerates the standard classification of the varying levels of DR and are found in the provided table (p.23).1-3
Most learned the clinically significant macular edema classification, however, the classification of DME has changed to reflect the evolution in diagnostic devices. Typically, DME is referenced as:4
- Non-central involved: retinal thickening in the macula that does not involve the center subfield zone that is 1 mm in diameter
- Central-involved: retinal thickening in the macula that does involve the central subfield zone.
DIAGNOSTIC DEVICES
The available diagnostic devices for diabetic eye disease include:
- Fundus photography. As evidenced by the definition of the different levels of DR from the ETDRS, color fundus photography combined with a dilated fundus examination remains the “gold standard” in monitoring patients who have diabetes. A color fundus photograph can show the different pathology of DR (hemes, intraretinal microsvascular abnormality, exudates) and allow for proper staging of the disease.
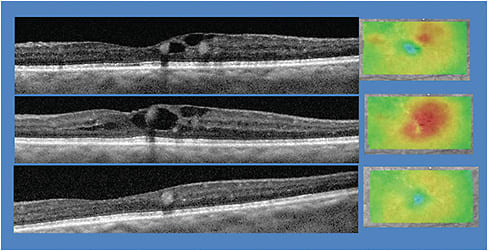
- SD-OCT. This technology acquires scans of the macula and choroid, providing an unprecedented view of the macular anatomy, enabling subtle cases of DME to be detected earlier. There is consensus that the central retinal thickness measured with SD-OCT is the reference standard for the assessment of DME.5
- OCT-A. This device acquires angiography-like images without the intravenous injection of fluorescein dye. Specifically, the technology reveals valuable clues as to the status of the vascular system of the retina, including areas of subtle neovascularization and hemes that might be missed on simple observation.
Electrophysiology testing, meaning visually evoked potentials and pattern electroretinography, are also available. For additional information online, visit bit.ly/3hXcVyU . - Scanning laser ophthalmoscopy. Scanning laser ophthalmoscopy provides ultra-widefield imaging, also helping to identify and determine the clinical severity of DR and DME.6 Much like fundus photography, diabetic pathology is revealed and allows the condition to be staged. Unlike traditional fundus photography, a larger area is captured with a single image. A caveat: Despite our ability to obtain many of these widefield images through a non-dilated pupil, HEDIS and the Quality Payment Program’s MIPS require a dilated fundus examination annually. (Generally, it is a good idea to review the payer’s policies.)
- Fluorescein angiography (FA). Much like OCT-A, FA helps to identify areas of abnormal and leaking blood vessels that may not be as apparent on visual inspection alone.
- Artificial intelligence (AI). Currently, there are two FDA-approved solutions for the detection of DR: IDx-DR (Digital Diagnostics) and EyeArt (Eyenuk).
The IDx-DR has 87.2% sensitivity, 90.7% specificity and 96.1% imageability in the detection of more than mild DR (mtmDR) and/or DME.7 Additionally, the AI device has a sensitivity of 97.6% for patients requiring immediate referral for treatment of DR and/or DME. The IDx-DR is approved for patients older than age 22 who have diabetes and have not been previously diagnosed with DR for the autonomous screening and detection of mtmDR or DME.
EyeArt has a refer rate of 91.3% sensitivity and 91.1% specificity for mtmDR and/or DME.8 For patients with mtmDR and/or DME, the sensitivity is 98.5%. EyeArt’s indication is for automatically detecting mtmDR and vision-threatening DR (severe nonproliferative DR or proliferative DR and/or DME) in eyes of adults diagnosed with diabetes who have not been previously diagnosed with mtmDR. CPT code 9255X relates to the use of these devices and will be released in January 2021.

PARTING THOUGHTS
As primary eye care providers, we can encounter seemingly asymptomatic patients and find evidence of retinopathy that leads to the initial diagnosis of diabetes. Also, we can expand our care of these patients to include blood glucose testing when diabetes is suspected. This test is CLIA-waived and easily performed, where allowed by state scope of practice. Further, we can take out our prescription pad or configure the EHR to order the HbA1C test. This can be sent with the patient to their nearest testing lab, if they don’t have a primary care doctor (PCP). Instructions should include having the results sent back to us.
Once results are received, appropriate management includes communication with the patient’s PCP or establishment with one. Timely communication with PCPs helps cement optometry’s place amongst the primary health care professions and demonstrates our value. Finally, we should consider downloading and reading the AOA’s, Eye Care of the Patient with Diabetes Mellitus, Second Edition (available at bit.ly/AOADiabetes2 ). It has many actionable items to enhance our optometric care of these diabetic patients. OM
| NONPROLIFERATIVE DIABETIC RETINOPATHY (NPDR) | |
| Mild NPDR | At least one retinal microaneurysm (Ma). Only hemorrhages/microaneurysm (H/Ma) are present, and the severity of the H/Ma is less than that depicted in the ETDRS standard photograph 2A. |
| Moderate NPDR | H/Ma greater than that depicted in ETDRS standard photograph 2A in one to three retinal quadrants OR soft exudates, venous beading (VB) and intraretinal microvascular abnormalities (IRMA) may be present to a mild degree. |
| Severe NPDR | Based in the 4-2-1 rule (any one of the following): → H/Ma ≥ ETDRS standard photograph 2A in four retinal quadrants → Definite VB in two or more retinal quadrants → Prominent IRMA (≥ ETDRS standard photograph 8A) in at least one quadrant |
| Very Severe NPDR | Two or more criteria (4-2-1 rule) are met, and there is an absence of neovascularization. |
| PROLIFERATIVE DIABETIC RETINOPATHY (PDR) | |
| PDR | Neovascularization of the Disc (NVD) or neovascularization elsewhere (NVE) |
| High-Risk PDR | Presence of at least 3 of 4 risk factors for severe visual loss: → Presence of pre-retinal or vitreous hemorrhage → Presence of new vessels → Presence of new vessels on or near the disc (NVD) → Presence of moderate or severe new vessels (neovascularization ≥ standard photograph 10A or NVE ≥ 1/2 disc area) |
| DIABETIC MACULAR EDEMA (DME) | |
| Clinically Significant Macular Edema (CSME) | |
| One or more of the following must be present: → Thickening of the retina ≤500 microns (1/3 disc diameters) from the center of the macula → Hard exudates ≤500 um (1/3 DD) from the center of the macula, with thickening of the adjacent retina → A zone or zones of retinal thickening ≥1 disc area in size, any part of, which is ≤1 DD from the center of the macula |
|
REFERENCES
- Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy From Stereoscopic Color Fundus Photographs—an Extension of the Modified Airlie House Classification. ETDRS Report Number 10. Ophthalmology. 1991;98(5 Suppl): 786-806.
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy. ETDRS Report Number 12. Ophthalmology. 1991;98(5 Suppl): 823-33.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for Diabetic Macular Edema: The Early Treatment Diabetic Retinopathy Study Report No. 4. Int Ophthalmol. Clin. 1987;27(4): 265-72.
- Evidence-Based Clinical Practice. Eye Care of the Patient With Diabetes Mellitus. Second Addition. American Optometric Association Web Site. https://www.aoa.org/AOA/Documents/Practice%20Management/Clinical%20Guidelines/EBO%20Guidelines/Eye%20Care%20of%20the%20Patient%20with%20Diabetes%20Mellitus%2C%20Second%20Edition.pdf . Accessed September 9, 2020.
- Virgili G, Meschini F, Casazza, G, et al. Optical Coherence Tomography (OCT) for Detection of Macular Oedema in Patients With Diabetic Retinopathy. Cochrane Database Syst Rev. 2015; 1:CD008081.
- Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic Ultra-wide Field Retinal Imaging Compared With the Dilated Standard 7-field 35-mm Photograph and Retinal Specialist Examination for Evaluation of Diabetic Retinopathy. Am J Ophthalmol. 2012;154(3)549-59.
- Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal Trial of an Autonomous AI-based Diagnostic System for Detection of Diabetic Retinopathy in Primary Care Offices. NPJ Digit Med. 2018;1:39. doi: 10.1038/s41746-018-0040-6. eCollection 2018.
- Bhaskaranand M, Ramachandra, C, Bhat S, et al. The Value of Automated Diabetic Retinopathy Screening With the EyeArt System: A Study of More Than 100,000 Consecutive Encounters From People With Diabetes. Diabetes Technol Ther. 2019;21(11): 635-643. doi: 10.1089/dia.2019.0164.





