A: AMD entails a family of diseases that have varied clinical characteristics, similar to glaucoma. This article will focus on unmasking the neovascular form (nAMD) and its imposters.
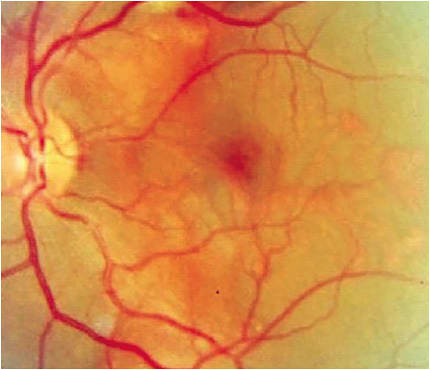
To start, let’s discuss nAMD’s clinical presentation. The condition is characterized by macular neovascularization, which typically begins in the choroid (CNV). (See Figure 1.) Other clinical features of nAMD include intraretinal or subretinal hemorrhage, retinal pigment epithelial detachment (RPED), lipid exudation, chorioretinal folds and eventual fibrovascular scar formation1,2,3. CNV, however, may occur in several conditions.
To distinguish the imposters from nAMD, we should consider patient demographics, a thorough history and identification of telltale clinical signs. Generally speaking, non-AMD causes of CNV tend to affect individuals at an earlier age. While nAMD is found primarily in elderly Caucasians, the imposters affect a wide range of ages and races.1 True age-related drusen are not found in these imposters. Here, we’ll discuss each of those distinguishing areas as they relate to common imposters.
POLYPOIDAL CHOROIDAL VASCULOPATHY
Polypoidal choroidal vasculopathy (PCV) was initially thought of as a subtype of nAMD. However, the lack of similar precursor clinical findings has led many clinicians to consider that PCV does not belong in the AMD spectrum. The CNV that eventually forms in PCV behaves differently from other forms of CNV; the overall visual prognosis also tends to be better than in other etiologies.2,4
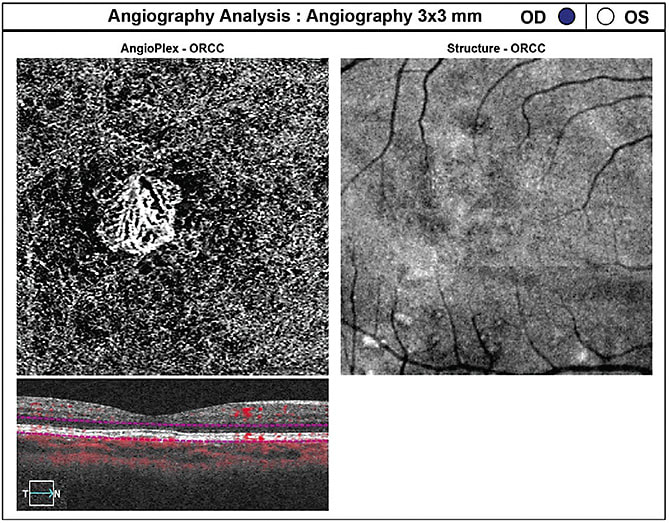
This CNV is a type 1 neovascular network (residing between the RPE and Bruch’s membrane) that exhibits an abnormal branching pattern of vessels characterized by aneurysmal dilations (referred to as polyps). (See Figure 2.) The disease can present as elevated orange nodules, recurrent hemorrhagic or serous RPE detachments (serosanguinous maculopathy), a large submacular hemorrhage or an occasional breakthrough vitreous hemorrhage.4 The lesions in PCV usually occur in the peripapillary area, however they may be found within the macula and mid-periphery.2
DEMOGRAPHICS. Few population-level studies exist on PCV; an accurate estimate of its overall prevalence is limited.4 The disease is now known to occur in all races, however it has a preponderance in darker pigmented individuals.
PATIENT HISTORY. Systemic hypertension may be associated with severe PCV visual loss.2
DIFFERENTIAL DIAGNOSIS. The differential diagnosis between typical nAMD and PCV can be challenging, however, several hallmark findings usually found in AMD, such as drusen, pigmentary changes and geographic atrophy, are uncommon in PCV. Because PCV has no universal accepted definition, many clinicians base the diagnosis on indocyanine green angiographic (ICGA) findings that show the presence of polypoidal dilatations. SD-OCT also may aid in the diagnosis of PCV: The polyps usually appear as inverted V-shaped anterior protrusions or dome-shaped elevations of the RPE and are known as “thumb-like RPED peaks.”
TREATMENT AND MANAGEMENT. Options for the treatment of PCV: Focal laser photocoagulation, verteporfin photodynamic therapy and anti-VEGF therapy (aflibercept, ranibizumab, and bevacizumab) can be used in isolation or in some combination.4 Anti-VEGF pharmacotherapies work by inhibiting the actions of VEGF, thus reducing angiogenesis and vascular permeability. Ocular photodynamic therapy (PDT) with verteporfin also may be considered when the CNV is found in the sub-foveal area. PDT uses a non-thermal laser to activate verteporfin, resulting in selective vascular occlusion. Standard laser photocoagulation exerts an action by coagulation necrosis.
DEGENERATIVE PATHOLOGICAL MYOPIA
Pathological myopia occurs because of excessive elongation of the globe that causes mechanical stretching and thinning of the RPE and choroid. Degenerative changes to Bruch’s membrane over time may eventually lead to CNV.
DEMOGRAPHICS. The prevalence of this disease varies among races and ethnic groups and is higher in women than in men.2 (Information on myopia demographics can be found in “Consider Demographics” at bit.ly/0620MyopiaDemo .)
PATIENT HISTORY. The incidence of myopic CNV can be up to 11.3% in individuals who have a diagnosis of pathologic myopia.1
DIFFERENTIAL DIAGNOSIS. The differential diagnosis between typical nAMD and pathological myopia is aided by the following indications: An eye that has > 6.00 D of myopia, a temporal crescent of atrophy adjacent to the optic nerve, macular gyrate atrophy and foveal linear breaks in Bruch’s membrane, known as lacquer cracks. At times, round areas of subretinal hyperpigmentation, known as a Fuchs' spot, may be found in the fovea, representing past subretinal hemorrhage or CNV.2 FA and SD-OCT/OCTA aid in documenting associated changes, such as myopic traction maculopathy and foveal schisis, that may occur.
TREATMENT AND MANAGEMENT. Treatment includes the off-label use of bevacizumab and ranibizumab. Recently, two large randomized, controlled clinical trials, the RADIANCE and MYRROR studies, showed favorable outcomes, designating anti-VEGF treatment as the current first-line therapy for myopic CNV. These pharmacotherapies work by inhibiting the actions of VEGF, thus reducing angiogenesis and vascular permeability.1 PDT with verteporfin also may be considered when the CNV is found in the sub-foveal area. PDT uses a non-thermal laser to activate verteporfin, resulting in selective vascular occlusion.
It is interesting to note that some CNV membranes from pathological myopia have been shown to remain stable over time without significant visual loss when no treatment is applied.2
ANGIOID STREAKS
Angioid streaks (AS) are full-thickness breaks in a thickened and calcified Bruch’s membrane. AS usually don’t cause visual impairment as long as the overlying retina remains intact. As in all conditions that contaminate Bruch’s membrane, CNV may occur along the track of AS. VA may be affected from the new blood vessel growth and subsequent fibrovascular scarring from subretinal hemorrhage.1-3
DEMOGRAPHICS. AS usually appear during the second decade of life, and their pathogenesis is unknown.3
PATIENT HISTORY. AS are idiopathic in 50% of cases, however, certain systemic conditions, such as Paget’s disease, Ehlers-Danlos syndrome, pseudoxanthoma elasticum and sickle cell anemia, may accompany them.2
DIFFERENTIAL DIAGNOSIS. The differential diagnosis between typical nAMD and AS can be aided by the use of FA, OCT and OCTA. AS appear clinically as bilateral red or brown irregular lines that radiate from the optic nerve to the macula and mid-peripheral fundus.2,3 In about 30% of patients, the streaks are associated with a diffuse mottling of the fundus that presents as a pattern called a peau d’orange appearance.3
TREATMENT AND MANAGEMENT. Intravitreal anti-VEGF medications, standard laser photocoagulation and ocular photodynamic therapy are all viable options for the treatment of CNV from AS. Anti-VEGF pharmacotherapies work by inhibiting the actions of VEGF, thus reducing angiogenesis and vascular permeability. PDT with verteporfin also may be considered when the CNV is found in the sub-foveal area. PDT uses a non-thermal laser to activate verteporfin, resulting in selective vascular occlusion. Standard laser photocoagulation exerts its action by coagulation necrosis.
OCULAR HISTOPLASMOSIS SYNDROME
Ocular histoplasmosis syndrome (OHS) presents with peripheral and central atrophic “punched out” chorioretinal scars (histo spots), peripapillary atrophy and maculopathy, presumably from previous exposure to a fungal Histoplasmosis capsulatum spore inhalation.3,5,6 The fungus, which is found in soil as a mold and in animals and birds as a yeast, is aerosolized when soil is disturbed during farming or when working with fowl, such as chickens.7,8 CNV may occur adjacent to one of the pre-existing scars within the macula area or adjacent to the peripapillary atrophy.
DEMOGRAPHICS. OHS is a leading cause of vision loss in Americans age 20 to 40.5 Additionally, those who work on a farm are at a greater risk for this diagnosis. Most cases of OHS occur in endemic areas of the United States, such as in the Ohio and Mississippi River Valleys.
PATIENT HISTORY. It has been proposed that the initial fundus presentation is from a prior systemic infection where H capsulatum was disseminated hematogenously from a primary lung infection to the choroid.3 More than 90% of OHS patients have a positive skin reaction to intracutaneous histoplasmin.
DIFFERENTIAL DIAGNOSIS. The differential diagnosis between typical nAMD and OHS can be aided by the use of FA, OCT-A and OCT. These imaging methods can help an optometrist to make an early diagnosis of the CNV adjacent to one of the pre-existing “punched out” scars, mentioned above.
TREATMENT AND MANAGEMENT. The first line of treatment currently for CNV in OHS is intravitreal anti-VEGF medication, which has been shown to be more effective and have less side effects than sub-macular surgery or thermal laser photocoagulation.9-11 Anti-VEGF pharmacotherapies work by inhibiting the actions of VEGF, thus reducing angiogenesis and vascular permeability. That said, the macular photocoagulation study shows the benefit of laser photocoagulation for extrafoveal and juxtafoveal CNV. Standard laser photocoagulation exerts its action by coagulation necrosis.
Additionally, PDT is of benefit for subfoveal CNV and refractory cases.3 PDT with verteporfin also may be considered when the CNV is found in the sub-foveal area. PDT uses a non-thermal laser to activate verteporfin, resulting in selective vascular occlusion.
CENTRAL SEROUS CHORIORETINOPATHY
Central serous chorioretinopathy (CSC) presents as a unilateral or bilateral circumscribed serous detachment of the neurosensory retina and, at times, the RPE. Subretinal precipitates of fibrin may be found below the detachment. In some cases, there is an inferior pooling effect due to gravity, leading to “gutters” of RPE alterations highly visible on FAF imaging. CSC is confined to the posterior pole. CNV, especially in older patients, may occur due to CSC’s chronic nature.
DEMOGRAPHICS. CSC is usually found in young and middle-aged adults.2 The Olmstead County Minnesota population study found an annual incidence of 9.9 in 100,000 in men and 1.7 in 100,000 in women.12
PATIENT HISTORY. The majority of CSC cases are considered idiopathic, however, other etiologies have been identified. There appears to be an abnormality of the RPE and choroid, in which fluid reabsorption from permeable choroidal vessels is impaired. Current evidence suggests a link with an active gastrointestinal helicobacter pylori infection. CSC has been associated with stress and type A personality, pregnancy, systemic lupus, hemodialysis, organ transplantation and in those using corticosteroids.3
DIFFERENTIAL DIAGNOSIS. The differential diagnosis between typical AMD and CSC can be aided by FA, OCT-A, OCT and widefield ICGA. OCTA has a 58% greater chance of aiding in the discovery of CNV vs. conventional dye-based angiography.12
TREATMENT AND MANAGEMENT. Most CSC patients have spontaneous resolution of fluid within one to three months, with a recurrence rate of approximately 40% within one year of the episode.2,3 However, laser photocoagulation and photodynamic therapy may be considered if persistent fluid occurs beyond three to four months.2,3
RULE OUT MASQUERADERS
While nAMD is the most common cause of CNV, myriad conditions can present in a similar fashion. By considering patient age, race, ocular and systemic history, the clinician can narrow down the differential diagnosis. Ophthalmoscopic findings, along with modern imaging methods are invaluable in ruling out conditions that masquerade as nAMD. OM
REFERENCES
1. Age-Related Macular Degeneration Preferred Practice Pattern – Updated 2015. American Academy of Ophthalmology website. https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015 Updated Jan. 2015. Accessed March 12, 2018.
2. Oellers P, Lains I, Mach S, et al. Novel grid combined with peripheral distortion correction for ultra-widefield image grading of age-related macular degeneration. Clinical Ophthalmology. 2017;11:1967-1974.
3. Davey PG, Alvarez SD, Lee JY. Macular pigment optical density: repeatability, intereye correlation, and effect of ocular dominance. Clin Ophthalmol. 2016;10:1671-1678.
4. Owsley C, McGwin G, Cleark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123:344-351.
5. Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investigative Ophthalmology & Vision Science. 1995;36:718–729.
6. Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, et al. Evaluation of autofluorescence imaging with the scanner laser ophthalmoscope and the fundus camera in age-related geographic atrophy. American Journal of Ophthalmology. 2008;146:183-192.
7. Roberts PK, Baumann B, Schlanitz FG, et al. Retinal pigment epithelial features indicative of neovascular progression in age-related macular degeneration. British Journal of Ophthalmology. 2017;101:1361-1366.
8. Dansingani KK, Gal-Or O, Sadda SR, et al. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesion in the taxonomy of ‘expanded spectra’-a review. Clinical & Experimental Ophthalmology. 2017 Nov 25 [Epub ahead of print] doi: 10.1111/ceo.13114.
9. Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123:361-368.
10. De Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125:255-266.
11. Midena E, Pilotto E. Microperimetry in age: related macular degeneration. Eye (Lond). 2017;31:985-994.
12. Gao SS, Jia Y, Zhang M, et al. Optical Coherence Tomography Angiography. Investigative Ophthalmology & Vision Sciences. 2016;57:27-36.






