Ocular surface disease (OSD) is known to be both chronic and progressive, with negative impacts on productivity and mental health. It is important to recognize that dry eye disease (DED), one segment of OSD, encompasses a combination of inflammation, evaporation and neurosensory abnormalities, according to TFOS DEWS II. Additionally, co-morbidities, such as blepharitis, are often hard to distinguish and need to be treated concurrently.1
I offer the following treatment protocol that I employ to help you to navigate the options for mild, moderate and severe DED.
MILD DED
Therapies for mild DED address the chief concerns of “dryness,” vision fluctuations and eye fatigue, which also are early signs the tear film can no longer support the ocular surface.2 Other signs of DED onset include reduction in contact lens wear time and cosmetic use dropout.2 As such, our goal as optometrists in cases of mild DED will be to clean and protect:

Clean. The initial treatment step of “cleaning” includes lid hygiene, such as scrubs, wipes, etc. The reason: Inflammation from over-abundant microorganisms can interfere with the success of other OSD therapies, as topical anti-inflammatories will have to fight excess inflammation from microorganisms in addition to the viscous cycle from T-cell-mediated response to the ocular surface.
When dealing with Demodex, thickened meibum secretions and sluffed-off epithelial cells are direct food sources; they also increase Staphylococcus and Streptococcus populations.1 Demodex can be treated with nightly tea tree oil or okra-based lid hygiene every other day to daily, for mild cases.1,3-5
Protect. Next, interventions for mild DED should focus on developing healthier occupational and vocational habits, particularly when it comes to digital device use. Blink breaks at intervals of 10 to 20 minutes can help to prevent exposure.2,6 Blink reminder apps can be installed on computers and smart phones (see Table 1).
| IRRITATION | SOLUTION |
|---|---|
| Visual hygiene/blinking |
|
| Anti-inflammatory |
|
| Exposure |
|
| Blepharitis |
|
| Eye-cosmetic irritation |
|
Other interventions to address environmental stressors include the use of non-preserved artificial tears (NPAT) every few hours and a desk-top humidifier, when needed.2
It’s worth noting that oral supplementation using omega-3 and omega-6 ocular nutritional supplements also has been shown effective on digital device users, both in calming inflammation and boosting healthy tear production.7-9
If you suspect the patients’ eyes open when sleeping and are, thus, causing DED, nighttime ocular surface protection is required. Sleep masks and non-preserved gel drops can help here.1
Further, switching to cosmetics with eye health in mind also can aid DED patients in avoiding ocular surface irritation. Eye make-up brands can be vetted using online and phone apps (see Table 1).
Finally, artificial elongation of the eyelashes with growth serums or cosmetic procedures should be avoided during DED treatment, as a “wind-tunnel effect” can occur if lashes are too long.10
MODERATE DED
Moderate DED patients have often reached the point where symptoms start to interfere with daily life. Chief complaints may include dry, itchy eyes, red eyes and an inability to wear contact lenses throughout the day, among others. As such, our goal as optometrists will be to clean, calm and protect the ocular surface, while being aware of the possibility of concurrent skin or systemic health conditions, including rosacea, thyroid and autoimmune disease.2
Clean. If Demodex is apparent via cylindrical dandruff that is greater than a grade 2, an in-office 50% tea tree oil application or okra-based lid scrub can decrease Demodex loads in two office visits, spaced one month apart.3-5 In my experience, the results can be maintained with nightly lid hygiene, as mentioned above.
Calm. Before protecting against future DED issues, we must first calm the ocular surface. As many patients who present to the optometrist with a chief complaint of “dry eye” have already tried artificial tears prior to coming to the office, I recommend pro-actively asking the patient whether they have used OTC artificial tears and, if so, which ones.
The patient may also be experiencing the end of the vicious inflammatory cycle, as demonstrated by punctate epithelial erosions (PEE) or superficial punctate keratopathy (SPK) in moderate OSD.2 (See image below.) In such cases, soft steroids can interrupt the inflammatory response with dosing two to four times per day of loteprednol, loteprednol etabonate or fluorometholone over two to four weeks.1 A formulation of loteprednol etabonate was recently FDA-approved for the short-term treatment of DED. Optometrists should follow the short 2- to 4-week steroid course to preclude risks of increased IOP and decreased wound healing. In such cases where these are of concern, a chronic anti-inflammatory can be implemented in place of a topical steroid. Chronic anti-inflammatory options include cyclosporine 0.5% or 0.09%, or lifitegrast 5%.1,11 For extra anti-inflammatory support, moderate DED patients also can dose autologous serum eyedrops (ASED) or platelet-rich plasma (PRP) drops four to six times a day in place of an NPAT.12
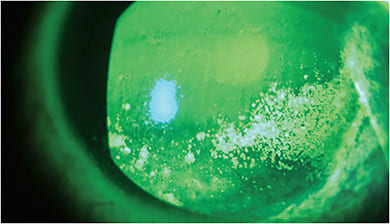
Protect. Once the inflammation is under control, optometrists can pivot to protect the ocular surface and keep inflammation low in moving forward:
- Commercial options for meibomian gland expression can be employed. Hand meibomian gland expression also has been shown to help those who find non-insurance covered treatments to be cost-prohibitive.13 This therapy removes build up and clears glands to allow for healthier production of meibum.
- Another in-office therapy for moderate DED is Intense Pulsed Light (IPL). IPL targets the irregular telangiectatic blood vessels seen in the eyelids of patients who have inflammatory DED, meibomian gland dysfunction (MGD) and ocular rosacea.14-17 When these blood vessels are closed-off, fewer pathways support an immune-mediated inflammatory response.
Outside of in-office treatments, environmental modifications, supplementation and good blinking practices that one might prescribe for mild DED, and as discussed above, also can be incorporated into a treatment plan for moderate DED. (See options in Table 2.)
| IRRITANT | SOLUTION |
|---|---|
| Blepharitis |
|
| Corneal/conjunctival Inflammation |
|
| MGD |
|
| Nocturnal lagophthalmos |
|
| Environmental |
|
| Eye-cosmetic irritation |
|
In those patients for whom sleep masks do not offer enough coverage for nocturnal lagophthalmos, switching to sleep goggles/shields and prescribing non-preserved, increased viscosity ointments can increase protection.1 If necessary and when appropriate, taping lids with medical tape can help those unable to sleep in masks.
SEVERE DED: HEAL-CLEAN-CALM-PROTECT
Patients who have severe DED will often describe severe pain, lack of daily functioning and embarrassment about the physical appearance of chronic redness and reflex tearing. Severe DED can manifest with ocular hyper-sensitivity or, conversely, with complete lack of sensation due to neurosensory abnormalities, such as neurotrophic pain. Corneal nerves can be unpredictable when they are in poor health. These patients also may report or confirm anxiety and depression associated with OSD.2 (The TFOS DEWS II therapy management and therapy report discusses possible psychological interventions.) As such, our goal as optometrists is to heal, clean, calm and protect.
Heal. For the severe DED patient to tolerate future in-office and home therapies, the initial course of treatment must target fast corneal/conjunctival healing. Amniotic membranes are an FDA-approved option for wound healing and work in a few days to stabilize the ocular surface.18 (See image below). Amniotic membranes are also shown to have an effect on corneal nerves.19 When amniotic membranes are unavailable, topical steroids and bandage contact lenses are options.
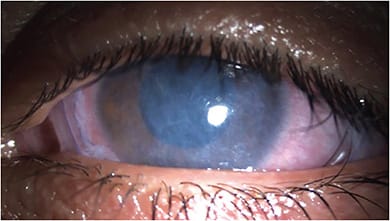
Clean. Severe concurrent blepharitis needs to be treated immediately, with in-office 50% tea tree oil or an okra-based lid scrub to prevent infective complications, such as lash loss, corneal inflammation and scarring.3-5
Calm. Once the ocular surface is clean, implementing in-office treatments (IPL/gland-expression) can help to knock out the chronic inflammatory response. IPL therapy may need to include one to two extra treatments for deeply imbedded inflammation/severe telangiectasia.14-17
Severe DED patients will follow the same treatment protocols as moderate disease patients (See Table 3) using chronic anti-inflammatory eye drops (cyclosporine 0.5% or 0.09% or lifitegrast 5%). ASED or PRP drops six to eight times a day is an option as well, as they include vitamin A and vitamin C, among other anti-inflammatory properties.12
| IRRITANT | SOLUTION |
|---|---|
| Blepharitis (same as moderate) |
|
| Corneal/conjunctival Inflammation |
|
| MGD |
|
| Nocturnal lagophthalmos |
|
| Environmental |
|
| Eye-cosmetic irritation |
|
| Autoimmune |
|
Protect. Patients who have unavoidable exposure (cases of nerve palsy, trauma, thyroid proptosis, post-blepharoplasty, etc.) may require scleral contact lenses and moisture eyewear to protect the ocular surface from excessive tear evaporation when the eyelids just won’t close.1 (More on this in “Contact Lens,” p.49.) Night care is also crucial and, again, requires sleep shields/goggles or taping in conjunction with lubrication.1
Additionally, severe DED can be associated with systemic autoimmune disease conditions, such as Sjögren’s syndrome, thyroid/Hashimoto’s disease and rheumatoid arthritis.2 This internal inflammation may be helped by comanaging the condition with the patient’s rheumatologist and endocrinologist.
For patients who do not make tears, they can benefit from punctal plugs, combined with chronic anti-inflammatory control, mentioned in the calming step for moderate DED.1
Keep in mind that due to neurosensory abnormalities, there may be patients who present with eye pain that persists, despite a clean and calm ocular surface with sufficient tears. Neuropathic pain is a diagnosis of exclusion. These patients may need comanagement with pain services and mental-health support.1
IMPROVE VISUAL FUNCTION AND SENSATION
OSD treatment success comes from a clean, calm and protected ocular surface. There are excellent choices for patients who are at all levels of DED, that can improve their visual function and sensation. OM
Additional therapies and therapeutic protocols for treating OSD can be found in Practicing Medical Optometry dry eye editions at optometricmanagement.com/practicing-medical-optometry .
REFERENCES
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report, Ocul Surf. 2017; 15(3): 575-628. doi: /10.1016/j.jtos.2017.05.006.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001. Epub 2017 Jul 20.
- Cheung IMY, Xue AL, Kim A, Ammundsen K, Wang MTM, Craig JP. In vitro anti–demodectic effects and terpinen-4-ol content of commercial eyelid cleansers. Cont Lens Anterior Eye. 2018;41(6):513-517. doi:10.1016/j.clae.2018.08.003.
- Islam MT. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother Res. 2019;33(1):72-80. doi: 10.1002/ptr.6212. Epub 2018 Oct 22. PMID: 30346086.
- Liu W, Gong L. Anti-demodectic effects of okra eyelid patch in Demodex blepharitis compared with tea tree oil. Exp Ther Med. 2021 Apr;21(4):338. doi: 10.3892/etm.2021.9769. Epub 2021 Feb 10.
- McMonnies CW. Incomplete blinking: Exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens and Anterior Eye. 2007; 30(1): 37-51. https://doi.org/10.1016/j.clae.2006.12.002 .
- Larmo PS, Järvinen RL, Setälä NL, et al. Oral Sea Buckthorn Oil Attenuates Tear Film Osmolarity and Symptoms in Individuals with Dry Eye. J Nutr. 2010;140(8):1462-8. doi: 10.3945/jn.109.118901.
- Sheppard JD, Singh R, McClellan AJ, et al. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca. Cornea. 2013; 32(10):1297-1304 doi: 10.1097/ICO.0b013e318299549c
- Molina-Leyva I, Molina-Leyva A, Bueno-Cavanillas A. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmol. 2017;95(8):e677-e685. doi:10.1111/aos.13428
- Amador GJ, Mao W, DeMercurio P, et al. Eyelashes divert airflow to protect the eye. J R Soc Interface. 2015 Apr 6;12(105):20141294. doi: 10.1098/rsif.2014.1294.
- Jerkins GW, Pattar GR, Kannarr SR. A Review of Topical Cyclosporine A Formulations—A Disease-Modifying Agent for Keratoconjunctivitis Sicca. Clin Ophthalmol. 2020;14:481-489. doi:10.2147/OPTH.S228070.
- Alio JL, Rodriguez AE, Ferreira-Oliveira R, Wróbel-Dudzińska D, Abdelghany AA. Treatment of Dry Eye Disease with Autologous Platelet-Rich Plasma: A Prospective, Interventional, Non-Randomized Study. Ophthalmol Ther. 2017;6(2):285-293. doi:10.1007/s40123-017-0100-z
- Kaiserman I, Rabina G, Mimouni M, et al. The Effect of Therapeutic Meibomian Glands Expression on Evaporative Dry Eye: A Prospective Randomized Controlled Trial. Curr Eye Res. 2021;46(2):195-201. doi: 10.1080/02713683.2020.1789663.
- Wu, Y., Li, J., Hu, M. et al. Comparison of two intense pulsed light patterns for treating patients with meibomian gland dysfunction. Int Ophthalmol. 2020 Jul;40(7):1695-1705. doi: 10.1007/s10792-020-01337-0.
- Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17(1):104-110. doi: 10.1016/j.jtos.2018.11.004.
- Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017;11:1167-1173. doi:10.2147/OPTH.S139894
- Craig JP, Chen YH, Turnbull PRK. Prospective Trial of Intense Pulsed Light for the Treatment of Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci. 2015;56(3):1965-70. doi: 0.1167/iovs. 14-15764.
- McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the Dry Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677-681. doi: 10.2147/OPTH.S162203
- John T, Tighe S, Sheha H, et al. Corneal Nerve Regeneration after Self-Retained Cryopreserved Amniotic Membrane in Dry Eye Disease. J Ophthalmol. 2017; 2017:6404918. doi: 10.1155/2017/6404918. Epub 2017 Aug 15.





