With the assistance of diagnostic imaging, optometrists can identify diabetes sooner and effectively manage these patients. What’s more, optometry’s participation is crucial: As of 2020, the CDC estimates that one in every 10 people in the United States has diabetes.1 Alarmingly, one in five are unaware they have it.1 Additionally, diabetes is linked with COVID-19 hospitalizations, and, it is estimated that one in three U.S. adults will have diabetes by 2050.2,3
So, just how much of an impact can optometry make? In 2019 alone, O.D.s identified the signs of diabetic retinopathy (DR) in 359,027 individuals who did not know they had diabetes.4 The best way to prevent visual impairment in DR is to detect, refer and treat proliferative DR and vision-threatening DR as early as possible, as well as communicate DR severity to primary care physicians to aid in the systemic management of the disease.
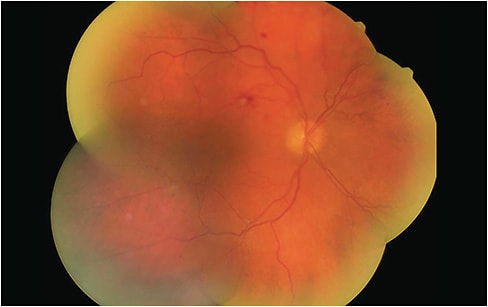
Understanding the pathophysiology of DR is imperative to appreciating the utility of the available imaging technologies. DR develops due to microvascular damage to the retinal blood vessels. The condition’s initial stages occur when chronic levels of high blood glucose lead to apoptosis of pericyte cells, which provide structural support and regulate the tight junctions and endothelial cell function of the retinal capillaries. As pericytes die, the retinal capillaries lose their ability to autoregulate, which leads to poor blood flow and reduced nutrient/oxygen deprivation to the inner retina. These initial changes to the vasculature occur in clinically “retinopathy-free” retinas on dilated examination.5 As such, the following technologies are beneficial to optometric practice. (See “Artificial Intelligence,” p.26.)
FUNDUS PHOTOGRAPHY
Fundus photography is a non-invasive modality that provides a stable retinal image of the otherwise moving and blinking patient. Also, the image can be enlarged and enhanced with software filters to visualize microvascular findings. Further, a fundus photograph is well suited to track changes over time, as photos from serial examinations can be compared.
Some items to keep in mind: Mild retinal edema and intraretinal vasculature changes can be missed on fundus photography, especially if the photos have poor image quality, due to artifacts or media opacification, such as cataracts. Also, standard devices provide a 30° to 45° field of view in a single image capture, typically encompassing the optic nerve and macula region. Since roughly 30% of DR occurs more peripherally than the posterior pole, several fundus photos are required to capture the entire retina.6 Widefield fundus cameras provide a 50° to 130° field of view and an ultra-widefield view up to 200,° making them ideal in capturing peripheral lesions. Patients who have peripheral DR lesions have a 3.2x higher risk of progressing in DR severity, and 4.7x higher risk of developing proliferative DR.6,7
SCANNING LASER OPHTHALMOSCOPY (SLO)
SLO uses low-powered red and green lasers to simultaneously scan the retina, producing two 2D images — the green (red-free) scan, which is more selective of the superficial neurosensory retina, and the red laser scan, which reflects the deep retinal layers, including the retinal pigment epithelium and choroid. SLO is advantageous in that it is easy to operate and does not require dilation. Because the images produced by SLO are not color-realistic images of the fundus, optometrists require education and practice in reading them.
Artificial Intelligence (AI)
AUTOMATED DEEP-LEARNING ALGORITHMS have been developed to improve the detection of DR. Two are currently available:

1 IDx-DR (Digital Diagnostics). This AI device produces a diagnostic interpretation and associated report, including care instructions aligned with the American Academy of Ophthalmology preferred practice pattern for diabetic retinopathy (DR).
It is intended to automatically detect more than mild DR in adults (22 years of age or older) diagnosed with diabetes who have not been previously diagnosed with DR, and it is indicated for use with the Topcon NW400.
Khadija S. Shahid, O.D., M.P.H., clinical assistant professor, Department of Ophthalmology and Visual Sciences, Carver College of Medicine, University of Iowa, says of the device: “IDx-DR has been used for diabetic eye exams for all of those patients with diabetes mellitus (DM) in our patient backlog. My clinic alone had a backlog of hundreds of patients, including those with DM who needed follow up to rule out vision-threatening DR and diabetic macular edema... IDx-DR allowed us to scan all of my canceled DM eye exams in a matter of weeks, rather than months to a year. Those identified as positive could then be prioritized into my patient schedule for a timely evaluation and prevention of vision loss...”

2 EyeScreen (Eyenuk, Inc.). This is a Human + AI Diagnostic Service for DR that assesses retinal images and provides an ICD-10 compliant report to the doctor, who also studies the images. The goal of the technology is to connect primary care/diabetes care clinics with eye care providers, so patients have increased access to DR screenings, less lengthy wait times and improved patient compliance upon receiving information regarding the screenings.
Paul Chous, MA, O.D., F.A.A.O., who has a practice specializing in diabetes eye care and education in Tacoma, Wash., and teaches a course on advanced topics in diabetes at Western University of Health Sciences, in Pomona, Calif., says of the device:
“I think it’s a nice auxiliary tool for the eye care provider, not a substitute,” he says. “It’s kind of a failsafe or a fallback position, in my view. It also helps eye care providers improve their DR grading skills, which is important when making decisions about patient management and referral to retina specialists. The key is acquiring solid images. As is the case with other diagnostic devices, its ability to detect retinopathy is only as good as the images that are submitted to it.”
AI further benefits O.D.s in that when used in primary care settings, it enables more appropriate and expeditious referrals to eye care providers. This increases patient access to care and reduces overall costs for the patient and healthcare system.
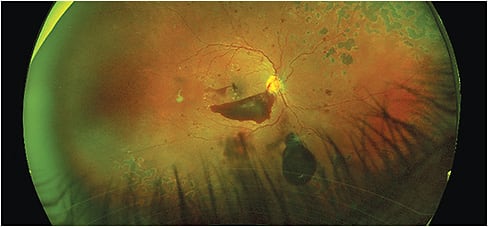
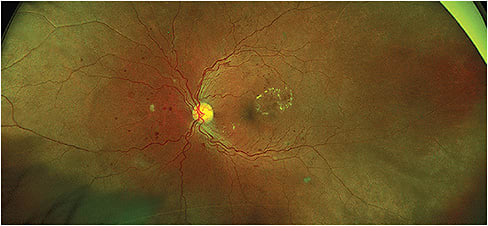
FLOURESCEIN ANGIOGRAPHY (FA)
FA requires the intravenous injection of fluorescein dye and may not be performed by an O.D., depending on state scope of practice law. FA is an ancillary procedure utilized to more accurately visualize diabetic retinal ischemia, blood flow dynamics and intraretinal edema to guide laser and injectable treatment decisions. FA, in addition to OCT/OCTA (discussed below), are necessary in addition to fundus photography, since the presence of hard exudates on fundus photography is suggestive of but not definitive of the diagnosis of intraretinal edema, and photographs are limited in their ability to depict retinal ischemia. As is the case with fundus photography, FA has greatly improved, with advances in widefield imaging that allow for increased visualization of peripheral vascular pathology.
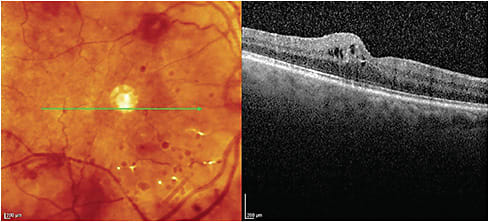
SPECTRAL DOMAIN (SD)-OCT
SD-OCT can aid in accurately quantifying retinal and choroidal thickness and identifying specific retinal layer pathology, such as intraretinal edema, in the inner plexiform layer from the deep capillary plexus or intraretinal edema in the retinal nerve fiber layer due to leakage from the superficial capillary plexus. FA/OCTA are necessary adjuncts, as they aid in visualizing other vascular changes, such as neovascularization, capillary drop-out and reduced blood flow.
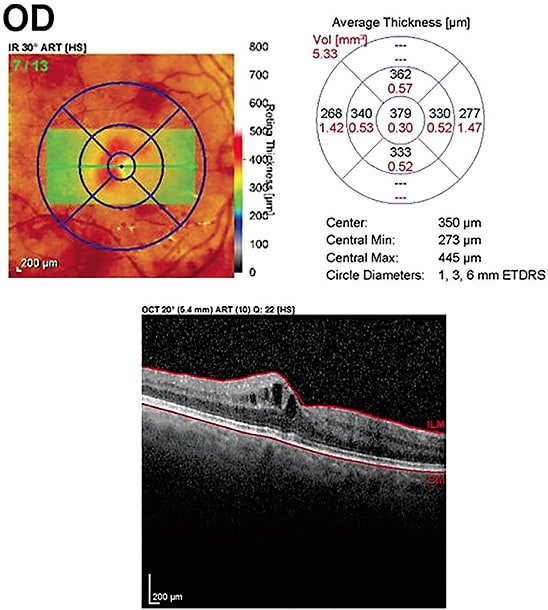
OCT ANGIOGRAPHY
This non-invasive device allows for the visualization of microvascular capillary details, blood flow and can aid in quantifying areas of non-perfusion and vessel density. Essentially, OCTA enables the optometrist to see smaller areas of neovascularization than might be visualized on fundus photograph or clinical examination, which can lead to expedited diagnosis and treatment. OCTA is currently limited in its ability to evaluate the peripheral retina.
WORKING TOGETHER
Due to the complicated pathophysiology of DR, no single diagnostic imaging tool can be used in isolation. Additionally, retinal imaging cannot aid in diagnosing nonretinal ocular complications of diabetes, like refractive error shifts, dry eye disease, cataracts and neovascularization of the iris and angle.8 It’s also worth noting that while recent advances in retinal diagnostic imaging technology are powerful in their ability to aid in identifying DR, none contain the doctor’s extensive knowledge, experience or valued bedside manner. Diagnostic imaging empowers clinicians to be able to detect retinal changes sooner and more accurately, but clinicians need to decide how that information is acted upon to deliver exceptional patient care: One truly needs the other. OM
References:
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed April 5, 2020.
2. Wargny M, Potier L, Gourdy P, e al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021 Apr;64(4):778-794.
3. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the U.S. adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010 Oct 22;8:29. doi: 10.1186/1478-7954-8-29.
4. American Optometric Association. This Diabetes Alert Day, valuable resources to help care for patients. https://www.aoa.org/news/clinical-eye-care/health-and-wellness/diabetes-alert-day?sso=y. Accessed April 12, 2021.
5. Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Research. 139 (2017): 7-14. Vision Res. 2017;139:7-14.
6. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587-2595.
7. Silva PS, Cavallerano JD, Hadad NMN, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015:122(5):949-956.7.
8. AOA Evidence-Based Clinical Practice Guideline. Eye Care of the Patient with Diabetes Mellitus. Second Edition. 2019. https://aoa.uberflip.com/i/1183026-evidence-based-clinical-practice-guideline-eye-care-of-the-patient-with-diabetes-mellitus-second-edition/0?m4=




