Because numerous medications can cause adverse effects to the retina, it is particularly crucial to obtain a thorough list of a patient’s prescription and OTC medications.
Here, we discuss these systemic medications, the retinal conditions associated with them (in order of how commonly they are seen) and how, specifically, to identify them, so we can work to preserve these patients’ vision. (See “Medication-Induced Cystoid Macular Edema”.)
Medication-Induced Cystoid Macular Edema
Numerous medications have been associated with cystoid macular edema (CME). We should keep in mind that even topical prostaglandin analogues can cause CME. In addition, the following medications can lead to CME: fingolimod, used in the treatment of multiple sclerosis, niacin and taxanes, imatinib and trastuzuman, which are chemotherapy treatments for various cancers, such as prostate cancer, lymphoblastic leukemia, chronic myeloid leukemia, gastrointestinal stromal tumors, myelodysplastic/myeloproliferative disease, breast cancer, stomach cancer, and esophagus cancer. Acutely CME can lead to blurred vision and metamorphopsia. Chronic CME may cause permanent visual acuity loss.
Treatment for medication-induced CME may include a topical or oral steroid or non-steroidal anti-inflammatory medications, intra-vitreal steroid or anti-VEGF medications and consideration of medication cessation.20,21,22,23 Prognosis is highly variable depending on the duration of CME and its response to treatment.
| Drug | Type of Toxicity/AE | *Number of Prescriptions | *Rank |
|---|---|---|---|
| HCQ | Bulls eye maculopathy | 5,406,190 | 129 |
| Tamoxifen | Crystalline maculopathy | 2,009,016 | 244 |
| Prednisone | CSR | 4,045,962 | 153 |
| Methylprednisolone | CSR | 27,057,494 | 21 |
| Topiramate | Myopic shift, retinal striae, uveal effusions, angle closure | 8,648,513 | 89 |
| Niacin | CME | 1,213,923 | 301 |
| Ethinyl Estradiol; Norethindrone (birth control pill) | RVO | 13,509,602 | 51 |
HYDROXYCHLOROQUINE
Hydroxychloroquine (HCQ), known as Plaquenil (Sanofi), is an anti-malarial drug commonly prescribed for autoimmune conditions, such as lupus.
- Retinal condition. Macular toxicity. Additional risk factors for toxicity are an HCQ daily dose > 5.0 mg/kg real weight; duration of use > 5 years; renal disease; liver disease; concomitant drug use (tamoxifen); and prior macular disease.1
- Identification. Advanced stages of HCQ-induced macular toxicity cause a pigmentary maculopathy with a bull’s eye appearance. Automated VFs plus SD-OCT are the primary screening tests, although multifocal electroretinogram (mfERG) can provide objective affirmation for VFs, and fundus autofluorescence (FAF) can reveal damage topographically. In Asian patients, tests should go beyond the central macula.1
Monitoring for irreversible maculopathy is critical. The American Academy of Ophthalmology recommends a baseline exam within one year of the patient initiating treatment, followed by annual monitoring after five years.1 With typical dosing (400 mg daily), five years marks nearing the potential lifetime toxic cumulative dose of 1,000 grams, usually reached by seven years of use.2
Yearly screening visits should include a dilated fundus exam, SD-OCT and 10-2 automated VFs. Current guidelines recommend 24-2 VF in Asian populations.1 Secondary tests, such as FAF and mfERG should be considered, if available (Figure 1).1
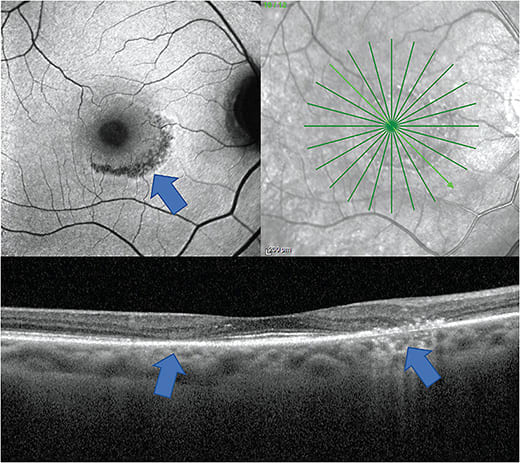
When HCQ-using patients present with possible signs of maculopathy, the prescribing doctor should be immediately informed, and discontinuation of HCQ and alternative therapy should be seriously considered. It is important to note that maculopathy can progress even after discontinuation.3 Those detected at early stages had fairly good outcomes, but those who had visible bull’s eye lesions tended to have poorer visual outcomes.
TAMOXIFEN
Tamoxifen is an anti-estrogen drug used to treat breast cancer.
- Retinal condition. Crystalline maculopathy. (See image at bit.ly/3EziakA .) The risk for retinal toxicity includes total cumulative dose and duration of treatment. Additionally, high body mass index and high cholesterol may be risk factors. Tamoxifen maculopathy can result in progressive central vision loss and color vision loss.
- Identification. A careful fundus evaluation can reveal crystalline deposits in the macula. O.D.s should evaluate the fundus and consider using OCT imaging to aid in identifying reflectile deposits or cystic retinal spaces.
A recent study suggests OCT imaging may increase detection over fundus evaluation alone.4 Of note: Crystalline maculopathy may be difficult to distinguish from macular telangiectasia type 2 (MT2).5 MT2 is a bilateral, although often asymmetric, retinal degenerative condition with findings most concentrated in the temporal juxtafoveal region. Findings can include crystalline deposits and cystic spaces on OCT, hence why it can mimic tamoxifen maculopathy. However, patients may also have juxtafoveal temporal pigmentary plaques and irregular vasculature. In addition, fluorescein angiography shows classic telangiectatic vessels, primarily in the temporal juxtafoveal region that leak in later stages.
An oncologist should be notified to consider discontinuing tamoxifen in those who develop crystalline maculopathy, especially if VA is effected. The drug can result in reduction of central VA and loss of color vision. Crystalline deposits and cystic spaces are often reversible with medication cessation, but the effects may also progress, even after stoppage.
PENTOSAN POLYSULFATE
Pentoson polysulfate is used to treat interstitial cystitis (swollen or irritated walls of the bladder).
- Retinal condition. Macular pigmentary alterations that can mimic AMD or pattern dystrophies. Distinguishing macular toxicity from AMD or one of its many mimickers is difficult, especially due to the limited data available on Pentoson polysulfate maculopathy. (See image at bit.ly/3zqy11b .) Any alteration to the outer retina or RPE seen on OCT or visualized with FAF should be given serious consideration. If the diagnosis is unclear, a referral for a second opinion is warranted. Central vision loss can occur from outer retinal degeneration in these patients.
- Identification. It has been suggested to obtain a baseline fundus examination, OCT and FAF to aid in picking up on RPE alterations. The clinical exam should pay careful attention to any pigmentary changes in the macula. When evaluating the OCT, O.D.s should pay careful attention to the outer retina and RPE, looking for any disruption, such as outer retinal or RPE atrophy. On the FAF, optometrists should be on the lookout for reticular-type alterations to the auto fluorescent signal that may include both hyper or hypo-autofluorescence. This should then be followed by yearly testing and repeat imaging beginning at five years of treatment.6
The association of maculopathy with pentoson polysulfate is relatively new, so prescribing physicians may not be aware of it. Therefore, it seems reasonable for O.D.s to correspond with prescribing physicians, so that they are aware of the association, the benefit of keeping patients on the lowest dose possible for the shortest duration of time and to inform them the patient will be monitored for ocular findings, which will then be reported to the prescribing doctor.
SYSTEMIC CORTICOSTEROIDS
Systemic corticosteroids, as is the case with topical corticosteroids, are anti-inflammatory agents.
- Retinal condition. Central serous chorioretinopathy (CSCR), associated with oral, nasal, topical, intra-articular or joint injectables.7
- Identification. The classic presentation is a pigment epithelial detachment (PED) with overlying serous detachment (Figure 2). Clinical exam is important, but OCT is highly useful in confirming findings. Also, FAF may aid in identifying regions of RPE disruption from previous episodes, especially in chronic or recurrent cases.
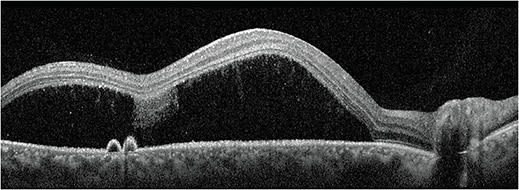
Acute CSCR cases can typically be monitored and will self-resolve with good visual outcome. Chronic or recurrent cases can lead to permanent vision loss. Therefore, in chronic or recurrent cases, patients should be referred for consideration of treatment, which may include focal laser, photodynamic therapy, oral aldosterone inhibitors or anti-VEGF injections. The benefit of anti-VEGF injections for serous detachments in CSCR is controversial, but they would be considered standard treatment for secondary choroidal neovascularization.7
MAPK/ERK KINASE (MEK) INHIBITORS
MEK inhibitors are chemo-therapy drugs that inhibit mitogen-activated protein kinase enzymes MEK1 and/or MEK2. This class of drugs is approved to treat certain types of melanomas and lung cancer and are in clinical trials for thyroid cancer, breast cancer, multiple myeloma and colon cancer.
- Retinal condition. Typically self-limiting serous retinal detachments. These have been described as “multifocal CSCR,” but unique characteristics (described below) from CSCR have led to the proposed designation of MEK inhibitor-associated retinopathy (MEKAR) rather than “multifocal CSCR.”
- Identification. MEKAR appears as multiple, bilateral serous detachments located in the fovea and extrafoveally along the vascular arcades (See images at go.nature.com/3nNZFmO ). OCT imaging is recommended to help identify these serous detachments and monitor them. OCT findings common in CSCR are often lacking in MEKAR, such as the presence of pigment epithelial detachments. In addition, a thick choroid is not associated with MEKAR as it is with CSCR.8 Those without center-involving serous detachments may be asymptomatic, but center-involving serous detachments often lead to symptoms of reduced vision and metamorphopsia.
If retinopathy occurs, optometrists should notify the prescribing physician of this occurrence. It is recommended to interrupt therapy in cases of symptomatic retinopathy and to resume therapy at a lower dose when symptoms resolve. Retinopathy can resolve after medication cessation, but there are reports of ongoing serous macular detachments even after the medication is discontinued.9
ESTROGEN
Estrogen is used in birth control medications and to treat amenorrhea and menopause, among other conditions associated with estrogen deficiency.
- Retinal condition. Retinal thrombotic events: namely retinal vein occlusions (RVOs). RVOs can lead to vision loss in numerous ways, including macular edema, macular ischemia and complications of retinal and iris neovascularization, such as neovascular glaucoma, traction retinal detachments and vitreous hemorrhage.
- Identification. RVOs include central retinal vein occlusions (Figure 3), hemi-retinal vein occlusions and branch retinal vein occlusions. These may present with dilated and tortuous veins, as well as intraretinal and flame-shaped hemorrhages, cotton wool spots and exudation along the affected vein(s). OCT aids in identifying macular edema. FA as well as OCT-A may help in identifying areas of vascular non-perfusion and retinal neovascularization.
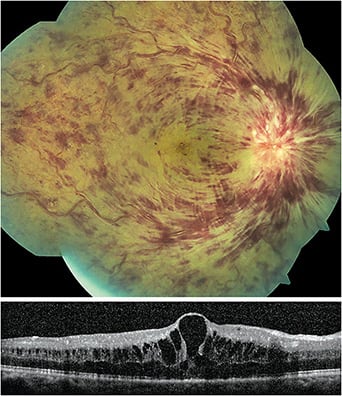
RVOs are associated with a variety of systemic disease and are most commonly seen in patients older than age 55 who have hypertension, diabetes, hyperlipidemia and in those who smoke. (When RVOs are seen in a younger, healthy patient, there should be increased concern for conditions that lead to a hypercoagulable or hyperviscosity state.)
RVO patients should be questioned regarding use of estrogen-containing drugs, particularly females of childbearing age. These patients should be co-managed with the prescribing physician to reduce the risk of future thrombotic events.10 Some RVOs self-resolve with good VA. The complications may require treatment, such as anti-VEGF, panretinal photocoagulation or vitrectomy, with variable outcomes.
LEUPROLIDE
Leuprolide is a chemotherapy drug used for breast, ovarian, endometrial and prostate cancers. Also, it can be used to treat non-cancerous conditions, such as endometriosis, uterine fibroids and infertility.
- Retinal condition. Thromboembolic events and RVO formation.11
- Identification. RVO presentation, prognosis and treatment are described above.
Communication with the prescribing doctor is necessary to determine the risk, benefits and alternatives to discontinuing treatment. Treatment of RVO complications are described above.
SULFA DERIVATIVES
Sulfa derivatives are prescribed for various bacterial infections. Examples are acetazolamide, topiramate and sulfa antibiotics.
- Retinal condition. Swelling of the ciliary body and forward rotation of the iris-lens diaphragm. This can lead to transient myopia, uveal effusions, secondary angle closures and retinal striations (Figure 4).12
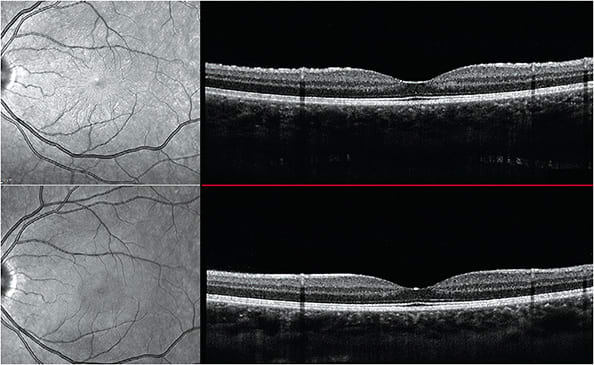
Figure 4. Top image shows subtle retinal striae on infrared reflectance image (left) and on the retinal surface (right). The patient had a myopic shift at the time. Bottom image shows resolution of striae post discontinuation of topiramate. The myopic shift also resolved. Image courtesy of Dr. Jessica Haynes. - Identification. Patients may present with transient myopic shifts, shallow anterior chamber, in-creased IOP, red, painful eye, choroidal detachments and retinal striations. Gonioscopy and AS-OCT may aid in evaluating the anterior chamber and angle anatomy. OCT and B-scan ultrasonography may help in evaluating the anatomy of the posterior segment to identify retinal striations or uveal effusions.
Medication discontinuation typically leads to resolution. Rises in IOP may need to be managed with topical drops, oral drugs or surgical intervention. Cycloplegia is helpful to relax the ciliary body and allow for posterior rotation.12,13
PHENOTHIAZINES
Phenothiazines are antipsychotics used to treat conditions, such as schizophrenia. Thioridazine and chlorpromazine are examples.
- Retinal conditions. Retinopathy, which is higher with thioridazine use. Thioridazine is not commonly prescribed due to its potential for heart arrhythmias. Patients taking doses of thioridazine greater than 800 mg/day are at particular risk, but retinopathy has been reported even in doses as low as 100 mg/day.14 For those taking high doses, retinopathy can occur acutely within three to eight weeks of use.14
- Identification. Retinopathy presents as widespread diffuse pigmentary alterations that may begin as mild pigmentary mottling posterior to the equator and progresses to geographic regions of RPE and choriocapillaris atrophy. Widefield fundus photography and FAF are useful in early detection. Color vision may be abnormal, and VF testing may reveal paracentral or ring scotomas.14
O.D.s should monitor patients during treatment. The medication should be discontinued if retinopathy occurs.14 Toxicity can occur acutely with symptoms of nyctalopia, dyschromatopsia and blurred vision.14 Without intervention, patients can suffer severe, permanent vision loss from diffuse RPE and outer retinal atrophy.14
INTERFERONS
Interferons help regulate the immune system, causing cells to increase their defenses against viral infection. Interferon use is common in patients who have hepatitis C, but these drugs also are used to treat multiple sclerosis and various cancers.
- Retinal condition. “Interferon” retinopathy. Patients with comorbidities, such as diabetes and hypertension, may be at greater risk.15
- Identification. “Interferon” retinopathy presents as cotton wool spots, retinal hemorrhages, macular edema and, rarely, artery or vein occlusion (Figure 5). Patients should be evaluated with dilated fundus examination. Ancillary testing, such as OCT, and color photographs may aid in monitoring the retinopathy.
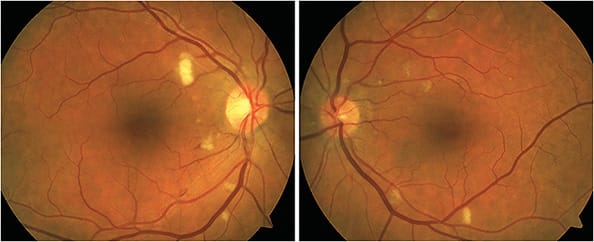
Retinopathy typically subsides with drug cessation. Coordination with the prescribing doctor is necessary in those who develop retinopathy, with consideration of therapy cessation in those who have sight-threatening lesions.15-17
GLITAZONES
Glitazones, including pioglitazone, treat diabetes.
- Retinal condition. Increased fluid retention causing macular edema.
- Identification. Evaluation for macular edema should include clinical evaluation for macular thickening and OCT imaging of the macula.
Especially in those who have additional signs of peripheral edema, medication discontinuation should be considered. Drug cessation tends to lead to quick resolution of peripheral edema and macular edema.18,19
PATIENT COMMUNICATION IS PARAMOUNT
Patients must always be educated regarding the importance of disclosing all medication — both prescription and OTC — to their eye care provider. Coordination with the prescribing doctor is crucial for good patient outcomes in those who present with retinal complications. OM
References
- American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy - 2016 https://www.aao.org/clinical-statement/revised-recommendations-on-screening-chloroquine-h . (Accessed September 7, 2021)
- Stokkermans TJ, Goyal A, Bansal P, Trichonas G. Chloroquine And Hydroxychloroquine Toxicity. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/pubmed/30725771 .
- Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014;132(9):1105-1112. doi:10.1001/jamaophthalmol.2014.1099
- Kim HA, Lee S, Eah KS, Yoon YH. Prevalence and Risk Factors of Tamoxifen Retinopathy. Ophthalmology. Vol 127. Elsevier Inc.; 2020:555-557. 2020 Apr;127(4):555-557. doi: 10.1016/j.ophtha.2019.10.038.
- Bommireddy T, Carrim ZI. To stop or not? Tamoxifen therapy for secondary prevention of breast cancer in a patient with ocular toxicity. BMJ Case Rep. 2016;bcr2015213431. doi: 10.1136/bcr-2015-213431.
- Hanif AM, Jain N. Clinical Pearls for a New Condition: Pentosan polysulfate therapy, a common treatment for interstitial cystitis, has been associated with a maculopathy. Review of Ophthalmology. July 10, 2019.
- Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118. doi:10.1016/J.PRETEYERES.2015.05.003
- Francis JH, Habib LA, Abramson DH, et al. Clinical and Morphologic Characteristics of MEK Inhibitor–Associated Retinopathy: Differences from Central Serous Chorioretinopathy. Ophthalmology. 2017;124(12):1788-1798. doi: 10.1016/j.ophtha.2017.05.038
- Booth AEC, Hopkins AM, Rowland A, Kichenadasse G, Smith JR, Sorich MJ. Risk factors for MEK-associated retinopathy in patients with advanced melanoma treated with combination BRAF and MEK inhibitor therapy. Ther Adv Med Oncol. 2020;12. doi:10.1177/1758835920944359
- Kirwan JF, Tsaloumas MD, Vinall H, Prior P, Kritzinger EE, Dodson PM. Sex hormone preparations and retinal vein occlusion. Eye. 1997;11(1):53-56.
- Federici TJ. Leuprolide acetate and central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2007;38(6):497-499. doi: 10.3928/15428877-20071101-09.
- Ryan EH, Jampol LM. Drug-induced acute transient myopia with retinal folds. Retina. 1986;6(4):220-223. doi: 10.1097/00006982-198606040-00005.
- Sankar PS, Pasquale LR, Grosskreutz CL. Uveal Effusion and Secondary Angle-Closure Glaucoma Associated with Topirimate Use. Arch Ophthalmol. 2001;119(8):1210-1211.
- Corradetti, G., Violanti, S., Au, A. et al. Wide field retinal imaging and the detection of drug associated retinal toxicity. Int J Retin Vitr 5, 26 (2019). https://doi.org/10.1186/s40942-019-0172-0
- Gaetani L, Menduno PS, Cometa F, et al. Retinopathy during interferon-β treatment for multiple sclerosis: case report and review of the literature. J Neurol. 2016;263(3):422-427.
- Feroze KB, Wang J. Interferon Induced Retinopathy. StatPearls Publishing; 2018. http://www.ncbi.nlm.nih.gov/pubmed/28722892 . (Accessed July 6, 2021.)
- Mohamed MAEB, Abd-El azeem Eed K. Retinopathy associated with interferon therapy in patients with hepatitis C virus. Clin Ophthalmol. 2012;6(1):1341-1345. doi: 10.2147/OPTH.S32469.
- Ryan EH, Han DP, Ramsay RC, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26(5):562-570. doi: 10.1097/00006982-200605000-00011.
- Oshitari T, Asaumi N, Watanabe M, Kumagai K, Mitamura Y. Severe macular edema induced by pioglitazone in a patient with diabetic retinopathy: A case study. Vasc Health Risk Manag. 2008;4(5):1137-1140. doi: 10.2147/vhrm.s3446.
- Makri OE, Georgalas I, Georgakopoulos CD. Drug-induced macular edema. Drugs. 2013;73(8):789-802.
- Saleh M, Bourcier T, Noel G, Speeg-Schatz C, Goucher D. Bilateral macular ischemia and severe vision loss following trastuzumab therapy. Acta Oncol. 2011 Apr;50(3):477-8. doi: 10.3109/0284186X.2011.555781.
- Newcott EK, Ellabban AA, Tavassoli S, Sallam A. Intravitreal bevacizumab and triamcinolone for treatment of cystoid macular oedema associated with chronic myeloid leukaemia and imatinib therapy. Case Rep Ophthalmol Med. 2015;2015:713868. doi: 10.1155/2015/713868.
- Masood I, Negi A, Dua HS. Imatinib as a cause of cystoid macular edema following uneventful phacoemulsification surgery. J Cataract Refract Surg. 2005;31(12):2427-2428. doi: 10.1016/j.jcrs.2005.10.029.





