Evidence of the rapid pace with which the field of myopia management is moving can be seen in the steady stream of published peer-reviewed journal articles and the release of new treatments.
Here, I discuss studies that have merit and review key recent studies on the current treatments available with some context.1
ANALYZING THE RESEARCH
When reviewing studies, the rigor with which they were conducted is of paramount importance. The following are considered highly desirable attributes of a myopia research study:
- Multiple years of data, as the slowing of myopia in the first 12 months is usually greater than in subsequent years.2
- Randomization and masking of both examiners and patients, when possible.3
- A control group matched by or analyzed, accounting for, age, ethnicity and other confounding factors.
- Refractive error is measured by cycloplegic autorefraction.3
- Axial length is measured, preferably, by non-contact, optical methods.2,3 When utilizing orthokeratology (ortho-k) as a therapy, refractive error is confounded by the intended corneal flattening, so axial length is usually the primary outcome measure. Although the ratio varies, 0.1 mm can be considered to equal 0.25 D.4-6
RESEARCH ON EMPLOYED THERAPIES
Spectacles. The Correction of Myopia Evaluation Trial (COMET) study shows a 3-year reduction in the progression of 0.20 D among PAL wearers compared to single vision wearers in children, with most of the effect observed in the first year of therapy.7 The slowing of axial elongation was 0.11 mm, consistent with the myopia control effect. As a post-hoc analysis appeared to show a larger treatment effect of PALs in children who have higher accommodative lag and esophoria, COMET2 was initiated. This study recruited only children who have high lags and esophoria.8 Participants were, again, randomized to receive either +2.00 D PALs or single vision lenses. Results reveal the mean 3-year myopia progression was –0.87 D and –1.15 D in the PAL and single vision groups, respectively — a difference of 0.28 D.
A study on the effect of executive bifocals on myopia shows mean 3-year progression was –2.06 D, –1.25 D and –1.01 D for single vision lenses, bifocals and prism bifocals, respectively. The corresponding axial elongation was 0.82 mm, 0.57 mm and 0.54 mm. This study’s young participants were randomized to single vision lenses, +1.50 D executive bifocals and +1.50 D executive bifocals with 3-∆ base-in prism in the near segment of each lens.9 Conversely, a previous study reports that the mean progression was –0.34 D, –0.36 D and –0.34 D per year for subjects wearing single vision lenses, +1.00 D bifocals and +2.00 D bifocals, respectively.10
When it comes to studies on spectacle lens technology designed to reduce peripheral hyperopic defocus, no differences were observed in the rates of progression when such designs were compared to the use of single vision lenses, although potential benefit was observed in younger children who have at least one myopic parent.11
This subgroup was subsequently evaluated in a study during which they wore a commercialized design (MyoVision, Carl Zeiss).12 Specifically, children younger than age 12 who had at least one myopic parent were randomized to single vision or MyoVision lenses. The results: Mean two-year progression in the treated group was -1.43 D compared to -1.39 D in the control group.
Track Progression
AN 8-YEAR-OLD MYOPIC FEMALE of Vietnamese ethnicity, who has two myopic parents, was treated with 0.02% atropine for a year. Her baseline refractive error was 2.55/-1.20D@135; OS -3.70/-0.55@140 OD, baseline axial length 24.51 mm, OS 24.51 mm.
Cycloplegic refractive error assessment (NK5001, Shin Nippon, Japan) was conducted at baseline and then every six months, whereas axial length measurements (Lenstar LS900, Haag-Streit) were performed more frequently, at three-month intervals.
Atropine 0.02% was used daily with one drop applied OU at bedtime. At three months, OU, a reversal or decrease in axial length compared to baseline was evident (see Figure 1); this pattern of reversal continued OS until six months. The findings correlated with the refractive error assessment where a slight myopic shift and a slightly hyperopic shift were observed OU. (See Figure 2). The change in axial length was less OS compared to OD over 12 months.
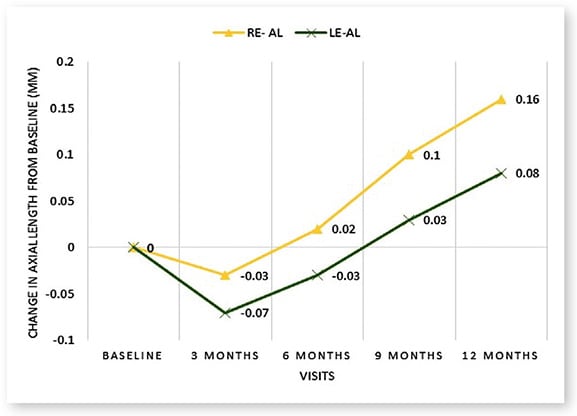

Pictured here: Change in axial length and spherical equivalent from baseline of the patient.
The combination of both axial length and refractive error assessments, in this case, provides an accurate, rapid and non-invasive means to track progression of myopia and yield confidence in the practitioner’s management method.
Lifestyle risk considerations taken into account: time outdoors outside of school, 0.5hrs/day, homework, 1.5hrs/day, reading and computer use, 1.5hrs/day.
— Padmaja Sankaridurg, Ph.D., Huy Tran, M.D.
Special thanks to Brien Holden Vision Institute for its contributions to this report.
Another spectacle lens designed for myopia management is the Defocus Incorporated Multiple Segments (DIMS) lenses, developed at the Hong Kong Polytechnic University.13 This design is comprised of a 9 mm central optical zone and a 33 mm annular zone that has multiple 1 mm segments containing a relative positive power of +3.50 D. A study on this lens reveals the mean myopic progression was –0.41 D in the DIMS group and –0.85 D in the control group. Additionally, mean axial elongation was 0.21 mm and 0.55 mm, respectively. The two-year study was made up of 160 children randomized to DIMS or single vision lenses. The DIMS lens is now available in many countries as Hoya’s MiyoSmart lens.
Yet another approach to spectacle lens therapy has been the use of 11 concentric rings formed by aspherical lenslets 1.1 mm in diameter.14 A study on this therapy shows the mean progression and elongation were –0.81 D and 0.36 mm in the single vision wearers compared to –0.27 D and 0.13 mm for the highly aspherical lenses (HAL) and –0.48 D and 0.25 mm for the slightly aspherical lenses (SAL) after one year of wear. The study was comprised of 170 children randomized to HAL, SAL or single vision lenses. The HAL design underlies Essilor’s Stellest lens, which is available in parts of Europe and Asia.
Finally, another annular design from SightGlass Vision is undergoing clinical trials, with promising one-year results reported at last year’s Annual Meeting of the American Academy of Optometry.
Atropine. This anticholinergic blocking agent has a long history of off-label use for myopia con-trol. Recently, it been subjected to randomized clinical trials.
In the Atropine in the Treatment of Myopia (ATOM) study in Singapore, subjects included myopic children ages 6 to 12.15 Children received either monocular 1% atropine or vehicle eye drops nightly for two years. The result: Mean myopia progression and axial elongation in the atropine-treated eyes was -0.28 D and -0.02 mm, respectively, compared with –1.20 D and 0.38 mm in the control eyes. After the clinical trial, treatment was ceased and subjects followed for an additional year.16 The atropine-treated group showed a one-year progression of -1.14 D — similar in magnitude to the two-year change in the control eyes. Given this rebound, the study’s investigators evaluated lower doses of atropine in ATOM2, sans a control group.17 This study was made up of 400 myopic children ages 6 to 12 who were randomly assigned to bilateral, nightly 0.5%, 0.1% and 0.01% atropine for two years. Results reveal the mean myopia progression was -0.30 D, -0.38 D and -0.49 D, respectively. The contrast between the myopia progression in this study and that of the progression of -1.20 D in the placebo group in the original ATOM study have led to widespread use of 0.01% atropine, particularly in East Asia. Specifically, a report shows that 345 out of 493 pediatric ophthalmologists surveyed who manage myopia use pharmacological therapy, with atropine 0.01% being the most popular at 63.4%.18 Of note: 0.01% atropine did not influence axial elongation.19 In ATOM2, the mean elongation was 0.27 mm, 0.28 mm and 0.41 mm in the 0.5%, 0.1% and 0.01% groups, respectively.17 The corresponding elongation in the control eyes in ATOM was 0.38 mm.15

The Low-concentration Atropine for Myopia Progression (LAMP) study, which randomized 438 myopic children ages 4 to 12 to 0.05%, 0.025% and 0.01% atropine or placebo for one year, shows mean myopia progression was –0.27 D, –0.46 D, –0.59 D and –0.81 D in the 0.05%, 0.025% and 0.01% atropine, and placebo groups, respectively, with corresponding mean axial elongation of 0.20 mm, 0.29 mm, 0.36 mm and 0.41 mm.20
In the past year, four additional clinical trials of 0.01% atropine have been published with results similar to LAMP.21-24
Ortho-k. The first randomized clinical trial of ortho-k, in which 102 children were assigned to either the specialty contact lens or spectacles, reveals the mean axial elongation was 0.36 mm in the ortho-k group and 0.63 mm in the control group.25
Another study on ortho-k shows the increase in axial length was 0.99 mm for the ortho-k group and 1.41 mm for the control group, based on five-year data regarding 43 subjects.26
Further, a study on 30 patients at seven years, reveals the seven-year change in axial length was 0.91 mm in the ortho-k group and 1.36 in the control group.27
Most marketed ortho-k lenses are not approved for myopia control; their use for this purpose is considered off label, although there are exceptions, particularly in Europe.
Soft contact lenses. Studies show that soft contact lenses that have a central distance zone and increased positive power in the periphery can significantly slow myopia progression. (Discussion here is limited to commercially available designs with at least two years of data. Few of these lenses are approved for myopia control in the United States and Europe.) These studies:
A two-year, five-arm clinical trial, wherein children were randomized to single vision soft contact lenses, two soft lens designs that imposed myopic defocus across the peripheral and central retina or two extended depth of focus (EDOF) soft lenses incorporating higher-order aberrations to modulate retinal image quality, shows the single vision group progressed by -1.12 D, while all other groups progressed ranging from -0.78 D to -0.87 D.28 The corresponding axial elongation was 0.58 mm in the single vision group compared with 0.41 mm to 0.46 mm in the other subsequent groups. Though not currently available in the United States, one of the EDOF lens designs is available in some markets from Mark’ennovy, marketed as the MYLO lens. It is CE marked for myopia management.
A three-year randomized clinical trial of the MiSight (CooperVision) 1-day dual-focus soft contact lens, in which myopic children were randomized to either the MiSight lens or Proclear 1-day spherical lens (both omafilcon A) worn on a daily disposable basis, reveals mean progression was -0.51 D in the MiSight group and -1.24 D in the control group, while axial elongation was 0.30 mm in the MiSight group and 0.62 mm in the control group.29
Finally, a double-masked clinical trial of CooperVision’s Biofinity D multifocals in 294 children, in which participants were randomly assigned to wear high add power (+2.50 D), medium add power (+1.50 D) or single vision soft contact lenses, shows three-year myopia progression was –0.60 D, –0.89 D and –1.05 D, respectively. Corresponding axial elongation was 0.42 mm, 0.58 mm and 0.66 mm.
Additional Resources
 Newsletters, such as the new Mastering Myopia, can help keep you up to date.
Newsletters, such as the new Mastering Myopia, can help keep you up to date.
 Events, such as the Global Myopia Symposium, can provide education on myopia.
Events, such as the Global Myopia Symposium, can provide education on myopia.
 Industry groups, such as the Global Myopia Alliance Coalition, can provide resources.
Industry groups, such as the Global Myopia Alliance Coalition, can provide resources.
 Non-profits, such as Brien Holden Vision Institute, can be an excellent resource as well.
Non-profits, such as Brien Holden Vision Institute, can be an excellent resource as well. BUCKLE UP
This ride is moving so quickly that seatbelts are highly recommended. Three companies are conducting FDA trials of low-concentration atropine and expect activity for contact lenses and spectacles soon. Stay tuned to future editions of “Myopia Mythbusters” in Optometric Management for continuing coverage. OM
More on Myopia Management
Looking for more information about myopia management? We’ve got you covered:
REFERENCES
- Bullimore MA, Richdale K. Myopia Control 2020: Where Are We and Where Are We Heading? Ophthalmic Physiol Opt. 2020;40(3):254-270. doi: 10.1111/opo.12686.
- Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in Myopia Control. Prog Retin Eye Res. 2021;83:100923. doi: 10.1016/j.preteyeres.2020.100923.
- Wolffsohn JS, Kollbaum PS, Berntsen DA, et al. IMI - Clinical Myopia Control Trials and Instrumentation Report. Invest Ophthalmol Vis Sci. 2019;60(3):M132-M160. doi: 10.1167/iovs.18-25955.
- Cruickshank FE, Logan NS. Optical ‘Dampening’ of the Refractive Error to Axial Length Ratio: Implications for Outcome Measures in Myopia Control Studies. Ophthalmic Physiol Opt. 2018;38(3):290-297. doi: 10.1111/opo.12457.
- Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-Year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci. 2019;96(8):556-567. doi: 10.1097/OPX.0000000000001410.
- Hyman L, Gwiazda J, Hussein M, et al. Relationship of Age, Sex, and Ethnicity with Myopia Progression and Axial Elongation in the Correction of Myopia Evaluation Trial. Arch Ophthalmol. 2005;123(7):977-87. doi: 10.1001/archopht.123.7.977.
- Gwiazda J, Hyman L, Hussein M, et al. A Randomized Clinical Trial of Progressive Addition Lenses Versus Single Vision Lenses on the Progression of Myopia in Children. Invest Ophthalmol Vis Sci. 2003;44(4):1492-500. doi: 10.1167/iovs.02-0816.
- Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-Addition Lenses Versus Single-Vision Lenses for Slowing Progression of Myopia in Children With High Accommodative Lag and Near Esophoria. Invest Ophthalmol Vis Sci. 2011;52(5):2749-57. doi: 10.1167/iovs.10-6631.
- Cheng D, Woo GC, Drobe B, Schmid KL. Effect of Bifocal and Prismatic Bifocal Spectacles on Myopia Progression in Children: Three-Year Results of a Randomized Clinical Trial. JAMA Ophthalmology. 2014;132(3):258-64. doi: 10.1001/jamaophthalmol.2013.7623.
- Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: A Randomized Clinical Trial. Part II. Final Report by the Patient Care Team. Am J Optom Physiol Opt. 1987;64(7):482-98.
- Sankaridurg P, Donovan L, Varnas S, et al. Spectacle Lenses Designed to Reduce Progression of Myopia: 12-Month Results. Optom Vis Sci. 2010 Sep;87(9):631-41. doi: 10.1097/OPX.0b013e3181ea19c7.
- Kanda H, Oshika T, Hiraoka T, et al. Effect of Spectacle Lenses Designed to Reduce Relative Peripheral Hyperopia on Myopia Progression in Japanese Children: A 2-Year Multicenter Randomized Controlled Trial. Jpn J Ophthalmol. 2018;62(5):537-543. doi: 10.1007/s10384-018-0616-3.
- Lam CSY, Tang WC, Tse DY, et al. Defocus Incorporated Multiple Segments (Dims) Spectacle Lenses Slow Myopia Progression: A 2-Year Randomised Clinical Trial. Br J Ophthalmol. 2020;104:363-8.
- Bao J, Yang A, Huang Y, et al. One-Year Myopia Control Efficacy of Spectacle Lenses with Aspherical Lenslets. Br J Ophthalmol. 2021;bjophthalmol-2020-318367. doi: 10.1136/bjophthalmol-2020-318367.
- Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the Treatment of Childhood Myopia. Ophthalmology. 2006 ;113(12):2285-91. doi: 10.1016/j.ophtha.2006.05.062.
- Tong L, Huang XL, Koh AL, et al. Atropine for the Treatment of Childhood Myopia: Effect on Myopia Progression after Cessation of Atropine. Ophthalmology. 2009;116(3):572-9. doi: 10.1016/j.ophtha.2008.10.020.
- Chia A, Chua WH, Cheung YB, et al. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-54. doi: 10.1016/j.ophtha.2011.07.031.
- Zloto O, Wygnanski-Jaffe T, Farzavandi SK, et al. Current Trends among Pediatric Ophthalmologists to Decrease Myopia Progression-an International Perspective. Graefes Arch Clin Exp Ophthalmol. 2018;256(12):2457-2466. doi: 10.1007/s00417-018-4078-6.
- Bullimore MA, Berntsen DA. Low-Dose Atropine for Myopia Control: Considering All the Data. JAMA Ophthalmol. 2018;136(3):303. doi: 10.1001/jamaophthal mol.2017.6638.
- Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (Lamp) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019;126(1):113-24. doi: 10.1016/j.ophtha.2018.05.029.
- Fu A, Stapleton F, Wei L, et al. Effect of Low-Dose Atropine on Myopia Progression, Pupil Diameter and Accommodative Amplitude: Low-Dose Atropine and Myopia Progression. Br J Ophthalmol. 2020;104(11:1535-1541. doi: 10.1136/bjophthal mol-2019-315440.
- Wei S, Li SM, An W, et al. Safety and Efficacy of Low-Dose Atropine Eyedrops for the Treatment of Myopia Progression in Chinese Children: A Randomized Clinical Trial. JAMA Ophthalmol. 2020;138(11):1178-1184. doi: 10.1001/jamaophthal mol.2020.3820.
- Hieda O, Hiraoka T, Fujikado T, et al. Efficacy and Safety of 0.01% Atropine for Prevention of Childhood Myopia in a 2-Year Randomized Placebo-Controlled Study. Jpn J Ophthalmol. 2021;65(3):315-325. doi: 10.1007/s10384-021-00822-y.
- Saxena R, Dhiman R, Gupta V, et al. Atropine for Treatment of Childhood Myopia in India (I-Atom): Multicentric Randomized Trial. Ophthalmology. 2021. February. doi: 10.1016/j.ophtha.2021.01.026.
- Cho P, Cheung SW. Retardation of Myopia in Orthokeratology (Romio) Study: A 2-Year Randomized Clinical Trialized Clinical Trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077-85.
- Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-Term Effect of Overnight Orthokeratology on Axial Length Elongation in Childhood Myopia: A 5-Year Follow-up Study. Invest Ophthalmol Vis Sci. 2012;53(7):3913-9.
- Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutieeerez-Ortega R, Sugimoto K. Long-Term Efficacy of Orthokeratology Contact Lens Wear in Controlling the Progression of Childhood Myopia. Curr Eye Res. 2017;42:713-20.
- Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia Control with Novel Central and Peripheral Plus Contact Lenses and Extended Depth of Focus Contact Lenses: 2 Year Results from a Randomised Clinical Trial. Ophthalmic Physiol Opt. 2019;39:294-307.
- Chamberlain P, González-Méijome JM, Logan NS, et al. A Three-Year Randomized Clinical Trial of Misight Lenses for Myopia Control. Optom Vis Sci. 2019 Aug;96(8):556-567. doi: 10.1097/OPX.0000000000001410.




