Consider these common clinical scenarios: a 42-year-old, mildly hyperopic and presbyopic female patient expresses a desire to wear contact lenses now that she is fully dependent on glasses. A 70-year-old, low myope, using several medications, presents wanting contact lenses for his weekend golf outings. A 10-year-old hyperopic astigmatic spectacle wearer who’s an avid gamer wants to “switch” to contact lenses.
Will peri-menopausal changes be a factor in the 42-year-old’s ability to successfully wear contact lenses? Will the medications the 70-year-old is taking, not to mention his advanced age, have a negative effect on his ability to wear contact lenses? Will the 10-year-old gamer be able to tolerate contact lens wear, given her six hours of digital device use daily?
While the optometrist’s initial consideration in contact lens selection is based upon refractive and lifestyle needs, the patients described above serve as an important reminder that ocular surface health should be given equal consideration to increase the likelihood of successful wear. What’s more, the Tear Film & Ocular Surface Society (TFOS) report on contact lens discomfort differentiates contact lens-related dryness and contact lens dry eye from contact lens discomfort. (See “Diagnose Contact Lens Discomfort,” p.48.) Specifically, it specifies that both refer to contact lens wearers who have pre-existing dry eye disease (DED), which may or may not be exacerbated by lens wear.1 Thus, the detection of DED in our new contact lens wearers is critical.
Here, I discuss the specific challenges to the ocular surface associated with contact lens wear, and the steps I take in a pre-fit ocular surface evaluation.
SPECIFIC CHALLENGES
A healthy ocular surface depends on the delicate balance of complex anatomic and physiologic components to fulfill the biological role of ocular protection and the provision of an optimal refractive surface. Contact lens wear can uniquely alter the homeostasis of the ocular microenvironment in several ways:
- Splitting the preocular tear film. The presence of a contact lens splits the preocular tear film. This can result in a reduced lipid layer thickness, decreased tear volume, and an increased tear evaporation rate.1 Contact lens discomfort may then arise in patients who are otherwise asymptomatic for DED and who present without pre-fitting clinical signs of tear film instability.
- Bulbar and tarsal conjunctiva exposure. The contact lens represents a foreign surface to which the bulbar and tarsal conjunctiva are exposed. Bulbar staining has been associated with an increase in dry eye symptomatology in contact lens wearers.2 Bulbar staining noted away from the limbus and peripheral to the edge of the contact lens can result from poor tear film coverage of the whole ocular surface in the presence of a contact lens.2 Specifically, frictional forces, creating mechanical micro-trauma may induce inflammatory and physical alterations in the tarsal conjunctiva as evidenced by lid wiper epitheliopathy (LWE).3,4
- LWE. The presence of LWE is greater and more severe in contact lens wearers, especially in contact lens wearers who complain of dry eye.5
- Contact lens-caused debris. The contact lens is a substrate for biologically burdensome organic and inorganic debris. While the increased prescribing of daily disposable and frequent replacement contact lenses has greatly reduced the clinical concerns for surface deposition in many patients, it is still a factor for the growing number of specialty lens wearers. Contact lens deposits impact vision and comfort and may also trigger inflammatory reactions, such as giant papillary conjunctivitis.6
ASSESSMENT BEFORE THE FIT
I find that an “outer to inner” approach to my examination is the most efficient and effective means to both identify and differentially diagnose DED. Here is my preferred testing sequence, which I realize may differ from others: (See “DED Diagnostic Items,” p.41.)
- Patient questionnaire. The determination of DED symptoms, if present, can be made through a patient questionnaire. Questionnaire examples include the Standard Patient Evaluation of Eye Dryness Questionnaire; the Ocular Surface Disease Index (OSDI); and the Dry Eye Questionnaire-5. These surveys alert the optometrist to the frequency, severity, and type of presenting symptoms. Additionally, these surveys establish a baseline score, which is helpful in the suspicion for diagnosis of DED and, should additional testing reveal clinical signs, the monitoring of the efficacy of treatment.
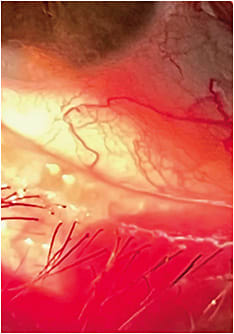
- Gross slit lamp observation. Using low illumination and low magnification, examining the structure and appearance of the external eye — the lids and lashes — as well as evaluating the completeness of the blink and the blink rate can reveal the presence of anterior blepharitis, Demodex, entropion, ectropion, or trichiasis and abnormal tear film quantity and quality, respectively. Also, corneal staining may be noted secondary to inverted lids or lashes; ectropion may cause tears to run off and reduce contact time of the tears to the ocular surface; and an incomplete blink pattern or reduced blink rate may predict contact lens discomfort.
- Tear chemistry analysis. Elevated tear osmolarity is a reported characteristic of dry eye disease.7 Additionally, studies suggest that hyperosmolarity increases with DED severity.8
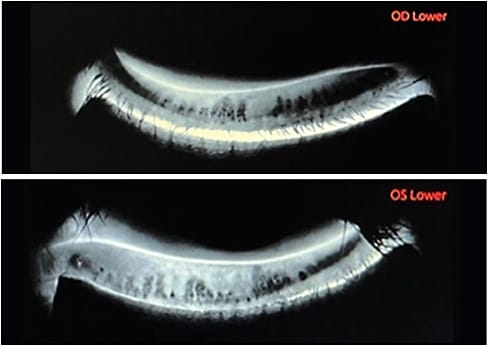
Matrix metalloproteinase (MMP-9) testing is helpful in differentiating early DED from more chronic and long-standing disease. Specifically, MMP-9, a protein marker elevated in the presence of chronic inflammation, is shown to correlate well with subjective symptoms evaluated by the OSDI, TBUT, Schirmer’s test, and conjunctival, as well as corneal staining. MMP-9 testing is also helpful as a baseline clinical metric in symptomatic patients and when repeated as necessary for assessing treatment efficacy.9 - Meibomian gland imaging. A variety of methods and devices exist for visualizing the structure of the meibomian glands. These range from simple lid eversion with a handheld light source, to stand-alone instruments and multifunctional modalities using infrared light. Imaging the glands reveals gland dilation, truncation, bending, and dropout,10 which are clinical signs of meibomian gland dysfunction (MGD), “a major cause of evaporative dry eye.”11
An additional benefit of meibomian gland imaging is patient education. Specifically, I find that showing patients images of their glands is the single most important factor in driving home the need for intervention. Furthermore, periodic re-imaging is equally important post-fitting, as contact lens wear has been associated with increased meibomian gland atrophy, as measured with meibography.1
Gross slit lamp observation is essential for examining the lids, lashes, and completeness of the blink and blink rate. Photo courtesy of Dr. Susan A. Resnick.
- Biomicroscopy/vital dye staining. Both sodium fluorescein and lissamine green (LG) staining are indicated in the contact lens pre-fitting examination. While both stains are useful, LG shows greater specificity for symptomatic subjects, particularly in contact lens wearers.12 Therefore, it is recommended that the examination of patients complaining of DED symptoms, in particular contact lens-wearing patients, include as routine, the examination of the conjunctiva utilizing LG.12 This relates back to the previous statement regarding the specificity of LG. The significance of sodium fluorescein staining in the contact lens wearer is not well understood. DED status has not been correlated with the presence of staining; however, studies have suggested a correlation with water content, wearing time, lens material, and deposition. Thus, a pre-fitting examination will help to determine whether future changes in staining presence or pattern is contact lens related.
Noted in the TFOS report on contact lens discomfort, there appears to be a weak link between contact lens discomfort and cor-neal staining. Furthermore, the conjunctiva is more closely linked to the development of contact lens discomfort. Bulbar conjunctival staining, typically viewed using LG, was found in some studies to be associated with contact lens discomfort, particularly soft lens edge-related staining.1 Finally, the instillation of fluorescein dye is also a simple way to measure TBUT. - Diagnostic meibomian gland expression. Quantifying meibum quality and expression are key components in MGD detection and stage grading.11 For instance, minimally altered expressibility and secretion quality would be recorded as Grade 1.11 Evaluating the number of expressible glands and the consistency of the expressed lipids on all new contact lens patients makes sense, as MGD may not always be obvious.13 Expression can be performed digitally (with a finger), using cotton-tipped applicators, various commercially available expressors or paddles, or with a specific device. (See sidebar) Regardless of which approach is used, I recommend that optometrists aim for a consistent testing and grading paradigm to achieve the most accurate data.
COVERING ALL THE BASES
The timely management of pre-existing ocular surface de-ficiencies, effective post-fitting troubleshooting, and the setting of patient expectations are dependent upon the optometrist’s comprehensive knowledge of patients’ anterior ocular profile. Employing a comprehensive, well-ordered clinical strategy to the pre-fitting evaluation is essential in achieving success in contact lens fitting. Just ask that 42-year-old, mildly hyperopic and presbyopic female patient, that 70-year-old, low myope, and that 10-year-old hyperopic astigmatic spectacle wearer.
DED Diagnostic Items*
BLINK ASSESSMENT
- iPEDA Blink Analyzer
MEIBOMIAN GLAND EXPRESSION
- Flexx MG Expressors (OcuSci)
- Mastrota Meibomian Paddle (Medi Instruments)
MEIBOMIAN GLAND IMAGING
- TearScience LipiScan (Johnson & Johnson Vision)
- TearScience LipiView II (Johnson & Johnson Vision)
- Meibomian Gland Evaluator (Johnson & Johnson Vision)
- Meibox (Box Medical Solutions)
- Oculus Keratograph 5M (Oculus)
- OPD Scan III (Marco)
- Orbscan III (Bausch + Lomb)
- Spectralis (Heidelberg)
TEAR CHEMISTRY
- Inflammadry (Quidel)
- Rapid Quantitative Tear Test for MMP-9 (AXIM Biotechnologies, Inc)
TEAR OSMOLARITY
- I-Pen Tear Osmolarity System (I-Med Pharma)
- TearLab Osmolarity System (TearLab)
TEAR QUANTITY
- OSData (Ophthalmic Resources)
- SMTube (OSDCare)
- Zone-Quick (Menicon)
*This sidebar will be updated, as needed, in the online version of this article.
REFERENCES
- Nichols JJ, Willcox MDP, Bron AJ, et al. The TFOS International Workshop on Contact Lens Discomfort: Executive Summary. Invest Ophthalmol Vis Sci. doi:10.1167/iovs.13-13212.
- Stapleton F, Golebiowski B, Skotnitsky C, et al. Corneal and conjunctival sensitivity in intolerant contact lens wearers. J Optom.. 2015;8(1):62-3. doi: 10.1016/j.optom.2014.05.004.
- Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom (Auckl). 2017;9:41-48. doi:10.2147/OPTO.S111130.
- National Research Council (US) Working Group on Contact Lens Use Under Adverse Conditions; Ebert Flattau P, editor. Considerations in Contact Lens Use Under Adverse Conditions: Proceedings of a Symposium. Washington (DC): National Academies Press (US); 1991. Treatment of Giant Papillary Conjunctivitis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK234094/ . Accessed June 9, 2022.
- Okumura Y, Inomata T, Iwata N, et al. A Review of Dry Eye Questionnaires: Measuring Patient-Reported Outcomes and Health-Related Quality of Life. Diagnostics (Basel). 2020;10(8):559. doi: 10.3390/diagnostics10080559.
- Allansmith MR, Korb DR, JV Greiner, AS Henriquez, MA, Simon, VM Finnemore. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol. 1977;83(5):697-708. doi: 10.1016/0002-9394(77)90137-4.
- Bunya VY, Langelier N, Chen S, Pistilli M, Vivino FB, Massaro-Giordano G. Tear osmolarity in Sjögren syndrome. Cornea. 2013;32:922-7. doi: 10.1097/ICO.0b013e31827e2a5e.
- Medscape. Dry Eye Disease (Keratoconjunctivitis Sicca) Workup. https://emedicine.medscape.com/article/1210417-workup#c9 Accessed une 1, 2022.
- Investigative Ophthalmology & Visual Science. ARVO Annual Meeting Abstract. Matrix-Metalloproteinase-9 (MMP-9) - Testing in Dry Eye Syndrome. https://iovs.arvojournals.org/article.aspx?articleid=2267299#:~:text=Conclusions%3A%20MMP%2D9%2Dtesting,inflammatory%20treatment%20in%20these%20patients . Accessed June 1, 2022.
- Vunnava KP, Shety N, Kapur KB. A review of meibography for a refractive surgeon. Indian J Ophthalmol. 2020;68(12):2663-2669. doi: 10.4103/ijo.IJO_2465_20.
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest Ophthalmol Vis Sci. 52:1938–1978. 10.1167/iovs.10-6997c.
- Guillon M, Maissa C. Bulbar conjunctival staining in contact lens wearers and non lens wearers and its association with symptomatology. Cont Lens Anterior Eye. 2005 Jun;28(2):67-73. doi: 10.1016/j.clae.2005.02.002.
- Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29(12):1333-45. doi: 10.1097/ICO.0b013e3181d4f366.




