As glaucoma is the leading cause of irreversible blindness worldwide, affecting more than 3 million people in the United States, with a projected 58% increase (4.2 million) by 2030, it is imperative we know the elements of a glaucoma consultation.1
Here, I provide them and offer suggested timelines for gathering data and monitoring for possible disease progression.
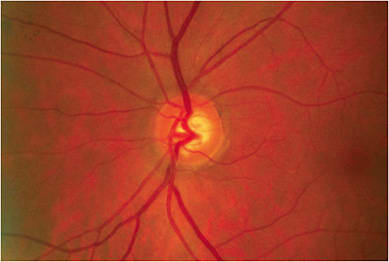
THE COMPREHENSIVE EYE EXAM
Many optometrists first identify a glaucoma or glaucoma suspect patient during the comprehensive eye examination. The action steps associated with this visit in my practice:
- Obtaining a thorough medical and ocular history, including that of family members. These histories should be acquired at every visit, as they can change. If the patient reports a family history of glaucoma, I document the maternal/paternal side and which family member has it. A family history of a parent or sibling having glaucoma usually increases the risk over other family members, such as an aunt or uncle, who may have glaucoma.2 Additionally, we should ask the patient what type of glaucoma (e.g., primary open-angle, angle closure, etc.) the family member has, so we can get an idea of what to possibly expect.
Pro Tips: In cases in which patients do not know exactly what eye conditions their family members may have, I have found it helpful to follow-up with, “does anyone in your family have an eye condition that they take eye drops for?” Further, for patients who are unsure of the type of glaucoma the family member has, I listen for descriptors, such as “normal pressure” or “pigment,” as they can provide defining clues. - Acquire VA.
- Obtain an IOP measurement. An accurate IOP measurement is vital for establishing a baseline in glaucoma risk assessment. While watching for elevated IOP is important, it is equally important to watch for IOP trends and not overlook low or normal-range IOP measurements. We should repeat IOP measurements if they are elevated or there is an outlier to the patient’s normal range (10 mmHg to 21 mmHg).
Pro Tip: It is often helpful to schedule follow-up appointments at different times during the day to gain an insight into how a patient’s IOP fluctuates daily.
THE WORKUP
I have found that it is time to consider initiating a full glaucoma workup when any of the following five criteria are identified during the comprehensive exam:
- Ocular hypertension, or an IOP measurement falling outside of an established patient’s “normal range.”
- Clinical signs of narrow anterior chamber angles, pigment dispersion syndrome, or pseudoexfoliation syndrome (even if the IOP and optic nerve appear “normal”).
- Optic nerves appear asymmetrical or present with notching, rim thinning, larger c/d ratio, or look suspicious overall for glaucomatous disease.
- History of ocular trauma or damage to the iris structures.
- Strong family history (two or more members) of glaucoma disease.
The timeline of when to initiate a glaucoma workup varies. I often schedule an initial glaucoma workup “next-available” (or in three to four months) for a baseline or low-risk glaucoma suspect. For patients who have many risk factors or glaucomatous-appearing optic nerves, I schedule a glaucoma workup sooner to decrease the risk of disease progression. My workup is comprised of:
- Tonometry. Consider repeating the IOP measurement for any glaucoma suspect patient using Goldmann applanation tonometry, as it is considered the gold standard of IOP assessment.3
- Gonioscopy. A baseline evaluation of the structures of the drainage system of the eye is done at the initial glaucoma workup and repeated at least once a year. The reason: Anterior chamber angles tend to narrow as we age, and drainage structures may change year to year, even in non-pigmentary or open-angle glaucoma cases. Many grading systems to assess the angle of the eye have been suggested, and there is no true right or wrong system. The grading system should describe both the width of the anterior chamber angle and the amount of pigment present. For additional information on the grading systems, see bit.ly/3K0epau .
Pro Tip: When documenting findings, stick with the grading system that works for you, keep it consistent, and make sure it is clear to interpret if other clinicians are reviewing your clinical data and notes. - Pachymetry. Using a pachymeter or AS-OCT are both acceptable modalities to assess the central corneal thickness. This assessment is crucial in every type of glaucoma to determine the correct IOP reading. If a patient has a thicker cornea (measured through pachymetry), that could indicate the pressure is actually lower than the reading. If someone has thin corneas, then the actual IOP may be higher than the IOP reading. If a patient has a surgery, such as LASIK, or a progressive corneal disease, such as keratoconus, causing a corneal change or a progressive corneal disease, we should consider repeating pachymetry to ensure we are appropriately assessing the accuracy of the IOP measurement.
- Corneal hysteresis (CH). This is another piece of data that can be helpful in a glaucoma workup to assess a patient’s risk level of glaucoma. Corneal hysteresis reflects the ability of the corneal tissue to dissipate energy. Low corneal hysteresis values often indicate an increased risk of developing glaucoma.4
- Slit lamp biomicrosopy. A thorough slit lamp biomicroscopy examination of both the anterior and posterior segments of the eye can provide insight into a patient’s risk level of glaucoma. I pay close attention to the anterior chamber angle, presence of pigment on the endothelium, the presentation of the iris, pseudoexfoliation material on the lens capsule, and I evaluate the optic nerve. Specifically, the optic nerve should be assessed for cup-to-disc ratios, cup-to-disc asymmetry, neuroretinal rim integrity, presence of a disc hemorrhage, peripapillary changes, notching, or bayoneting of the retinal vasculature.
- Dilated fundus eye (DFE) examination of optic nerve. I do a DFE to make sure I have a full view of the optic nerve and surrounding retinal nerve fiber layer (RNFL) areas. Sometimes, you can miss wedge defects or changes in surrounding RNFL layers if the view isn’t great with an undilated view. A dilated exam ensures I’m seeing more of the back part of the eye, which is advantageous for many reasons.
- Perimetry (HVF 24-2 or equivalent). Perimetry reveals the functionality of the ocular structures, making it a critical data piece of glaucoma management.5 Perimetry should be tested on each eye individually, and defects present should correspond with structural changes observed. A 24-2 standard automated perimetry is popularly applied perimetry to assess visual function. However, many new studies suggest that for some glaucomatous defects, additional perimeter-type assessments are warranted.6 Different patients may need different VF types, so we should not be afraid to test a 10-2 to fully assess glaucomatous damage to the macula area. Also, we should be aware that abnormalities in the VF may not always be associated with glaucoma and could indicate other underlying ocular or neurological pathologies. It should also be noted that there is sometimes a bit of a learning curve for patients taking a VF assessment, especially for the first time. If defects are present, I recommend repeating testing to confirm the defects and ensure they are repeatable.
- OCT. This is needed to measure the RNFL and ganglion cell complex (GCC). Assessing for thinning of the peripapillary RNFL and ganglion cell death via OCT can be helpful in both the early diagnosis of glaucoma and in monitoring disease progression. Most modern-day OCT devices allow for obtaining both scans easily. Some studies indicate that a loss of GCC may occur prior to RNFL loss.7
(For additional diagnostic devices to manage glaucoma patients, see “Additional Pieces of the Glaucoma Workup,” below.) - Gather data/provide patient education. After the glaucoma workup, we should analyze the conglomerate amount of data to establish a monitoring, treatment, or follow-up plan of action. All data gathered should be repeatable and reliable.
An important aspect of any glaucoma consultation is patient education. While a glaucoma suspect or glaucoma diagnosis may seem like an everyday item to us, for a non-clinician this can be life-changing news. Be professional, honest, and use easy-to-understand language. It is also important to explain what the condition is, how it can impact vision and overall ocular health, the prognosis, treatment options, needed lifestyle changes, and your plan of action in moving forward. We should allow time for patients to ask questions and to clarify any points of confusion, so they leave feeling informed and clear on a future plan of action. Educational handouts or a summary of the plan of action may also be helpful to distribute when the patient leaves the office. - Schedule repeat VF within one month if structural defects are present.
- Schedule repeat OCT scan within one month if RNFL thinning is noted.
- Repeat testing in one year if baseline glaucoma workup indicates monitoring and not initiating treatment.
Additional Pieces of the Glaucoma Workup
The following diagnostic devices are also available for the glaucoma workup and management:
Fundus camera. This enables imaging the optic nerve head for subtle alterations to the RNFL and the identification of Drance hemorrhages. Both may be unnoticed during dilation. For additional information, see bit.ly/3r4l7DT .
Pupilometers. The swinging flashlight test and automated infrared pupilometers can measure relative afferent pupillary defects and can be utilized to confirm the extent of asymmetric optic nerve head damage in glaucoma patients. For additional information, see bit.ly/32ZRhZp and bit.ly/3GunUwR .
SD-OCT/OCT-A. The former provides thickness measurements of the damaged neuroretinal tissue, RNFL, GCC, and neuroretinal rim. The latter uses motion contrast (comparison of sequential B scans of the same static retinal or optic nerve head area) to identify red blood cell movement, enabling a non-invasive, quantitative assessment of vascular health. For more information, visit bit.ly/3r4l7DT .
Sphygmomanometer. This is an important diagnostic measure for patients who have disease progression, despite adequate IOP control. Specifically, the in-office monitoring of blood pressure (BP) allows for the patient’s ocular perfusion pressure (OPP) calculation. Mean OPP = 2/3 DBP + 1/3(SBP – DBP) – IOP. Additionally, BP measurements are helpful both before and after prescribing a beta blocker or alpha-adrenergic agonist. For more information, see bit.ly/3r4l7DT .
Visual electrophysiology. Electrodiagnostic tests assess the retina and visual system to help detect functional issues before irreparable structural damage occurs. Visually evoked potentials (VEP) and pattern electroretinography (PERG) are useful for glaucoma suspects. VEP measures electrical activity in the visual cortex, with the amplitude and latency indicating healthy visual pathways vs. any dysfunction. PERG shows the amplitude and phase of retinal ganglion cell function and aids in the identification of the early signs of glaucoma. For additional information, see bit.ly/3n7WEMT .
THE INITIATION OF TREATMENT
If data suggests both structural and functional changes, initiating treatment to prevent progression is warranted. The initial choice of treatment should consider the patient, compliance of therapy capability, possible adverse effects, long-term treatment burden, and financial implications.
Anti-glaucoma drop therapy, ocular nutritional supplementation, and selective laser trabeculoplasty are first-line treatment options. Regarding the latter, it is important to explain that supplementation is meant to play an adjunct role and does not replace glaucoma medical treatment. Vitamin C, magnesium, vitamin B3, omega-3, and omega-6 fatty acids, and phenol compounds (i.e., ginkgo biloba, mirtogenol, resveratrol) may offer nutritional support in glaucoma cases. As always, we should have the patient consult with their primary care provider before starting any dietary supplement. For additional information on treatment, see bit.ly/3nbI0nR .
THE MONITORING OF PROGRESSION
After treatment has been initiated, the severity of the disease guides follow-up visits. Different patients need different follow-up schedules and treatment plans.
Typically, I have a patient return for a one-month follow-up if I am initiating, changing, or adding a treatment. My reasoning: A one-month follow-up allows enough time to assess treatment effectiveness without losing time and risking disease progression.
If the IOP measurement is acceptable at this one-month appointment, I have the patient return for follow-up again in three months. If a glaucoma condition continues to progress through either structural or functional assessment, adding adjunct topical or surgical options, such as laser trabeculoplasty, may be warranted.
For patients continuing antiglaucoma treatment, IOP measurements are typically done every three months, and OCT with VFs are done every six to 12 months to monitor for disease progression. Glaucoma patients are often seen at least four times a year, but one of those exams could also include a refraction or contact lens assessment component. (See “Suggest Refractive Correction Options for Glaucoma Patients,” p.28.)
Unstable/severe cases of glaucoma may need to be seen more frequently, while established and stable patients can be seen less. For efficiency, I typically have the following flow:
- Exam 1: Comprehensive eye exam to assess refractive needs.
- Exam 2: Three-month IOP check.
- Exam 3: Six months (or three months after IOP check) gonioscopy, pachymetry, dilated fundus exam, perimetry, OCT.
- Exam 4: Nine months (or three months after glaucoma testing exam) IOP check.
There is no right or wrong way to follow-up appointments. As long as the patient is well cared for, we should find the system that works for us as individuals. Perhaps, gonioscopy at the comprehensive eye exam is more efficient for your progression-monitoring flow? Multiple visits a year are also helpful in motivating the patient to be compliant with their prescribed treatments.
DUE DILIGENCE
Glaucoma is a complex ocular disease that requires a detailed consultation and a dynamic treatment approach. Early diagnosis is key, and both structural and functional changes of the optic nerve should be monitored. As primary eye care providers, we are in a unique position to play an important role in the diagnosis and treatment of glaucoma patients via a glaucoma consultation. OM
REFERENCES
- National Eye Institute. Glaucoma Data and Statistics. https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics/glaucoma-data-and-statistics . (Accessed Jan. 11, 2022.)
- ARVO Journals. Investigative Ophthalmology & Visual Science. ARVO Annual Meeting Abstract. May 2003. Significance of Family History of Glaucoma for Glaucoma Screening. https://iovs.arvojournals.org/article.aspx?articleid=2413995 . (Accessed Jan. 11, 2022.)
- Özcura F, Yildirim N, Sahin A, Çolak E. Comparison of Goldmann applanation tonometry, rebound tonometry and dynamic contour tonometry in normal and glaucomatous eyes. Int J Ophthalmol. 2015;8(2):299-304. doi:10.3980/j.issn.2222-3959.2015.02.15
- Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013;120(8):1533-1540. doi: 10.1016/j.ophtha.2013.01.032.
- Broadway DC. Visual field testing for glaucoma - a practical guide. Community Eye Health. 2012;25(79-80):66-70.
- Jung KI, Ryu HK, Hong KH, Kim YC, Park CK. Simultaneously performed combined 24-2 and 10-2 visual field tests in glaucoma. Sci Rep. 2021;11(1):1227. doi: 10.1038/s41598-020-80318-w.
- Bhagat PR, Deshpande KV, Natu B. Utility of Ganglion Cell Complex Analysis in Early Diagnosis and Monitoring of Glaucoma Using a Different Spectral Domain Optical Coherence Tomography. J Curr Glaucoma Pract. 2014;8(3):101-106. doi: 10.5005/jp-journals-10008-1171.




