Ms. Jones, a 65-year-old woman diagnosed with type 2 diabetes 10 years prior presents complaining of fluctuations in her vision and seeing more halos at night. Her blood sugar is poorly controlled on oral medication alone, and her last A1c was 10% two months prior to today’s appointment. What’s your next move?
When encountering such a patient, I would argue that most optometrists would be suspicious of diabetic retinopathy (DR) and, thus, perform, a dilated eye exam. Makes sense. After all, we are aware that glycemic control is an independent risk factor in developing DR and nephropathy.1
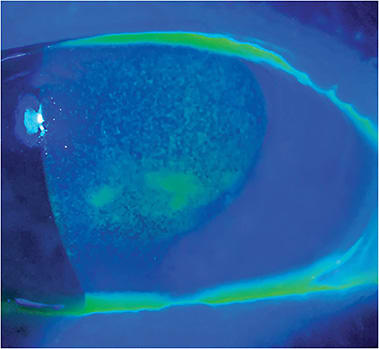
Guess what? Ms. Jones’ exam revealed 1+ nuclear sclerotic cataracts, along with 3+ diffuse superficial punctate keratitis, decreased TBUT, and 2+ meibomian gland dysfunction with turbid expression. No DR.
Here, I explain why the cornea should always be a consideration when managing diabetic patients, and how to treat patients whose corneas have been affected by diabetes.
WHY?
The cornea provides 75% of our eyes’ refractive power, and its epithelium provides a physical and immunological barrier to the environment.2 Without the cornea, even the retina becomes superfluous.
Innervated by the ophthalmic division of the trigeminal nerve, when stimulated, the cornea nerves are responsible for protective reflexes, including tear production and blinking, as well as trophic support to corneal epithelial cells and keratocytes. When these nerves are damaged, this can lead to compromised cornea renewal and healing, as well as a reduction in tear formation and tear film dysfunction.
Studies since the 1970s show decreased corneal sensitivity in diabetic patients.3,4 Additionally, there is a correlation between the severity of the diabetes vs. the increased incidence of damage to corneal innervation.4 On in vivo confocal microscopy of the cornea, studies reveal actual visualization of the changes to these patients’ nerve fiber density, length, branch density, and increased nerve tortuosity and thickness.4
In a setting of chronic hyper-glycemia, the glucose level is measurably increased in tears.5 This causes an elevated expression of advanced glycation end-product (AGE)-modified proteins leading to enhanced expression of nuclear factor-kappa B (NF-kb).5 NF-kb regulates cell proliferation and apoptosis, as well as the release of tumor necrosis factor alpha (TNF-a), activation. This leads to cytokine, chemokine, and proinflammatory release, which has been demonstrated to be involved in the pathogenesis of dry eye disease (DED).1,5,4 In addition, this inflammatory cascade can lead to a decrease in nerve growth factor and sphingolipid production, damaging corneal neurons.1,6,7
Also, in this same scenario of hyperglycemia, we see that insulin growth factor binding protein 3 (IGFBP3) is released and competes for binding sites to inhibit insulin growth factor 1 (IGF-1). At the same time, transforming growth factor beta 3 (TGFB3), epidermal growth factor receptor (EGFR), and ciliary neurotrophic factor (CNTF) are all decreased. Both occurrences cause reduced epithelial cell proliferation and increased apoptosis during the healing process of epithelial defects.
These changes are the underlying cause of both increased DED and neurotrophic keratitis (NK) in our patients who have diabetes. The fact is diabetes is considered one of the leading systemic risk factors for DED, with an incidence of 15% to 33% in patients older than age 65 who have diabetes.5 Additionally, The Beaver Dam Eye Study reports DED in approximately 20% of patients between the ages of 43 and 86 who have type 2 diabetes.8 Another study shows 53% of patients who had diabetes or borderline diabetes self-reporting clinically relevant dry eye.6 A correlation was found related to severity of DR in patients to an increased risk of DED when compared with patients who did not have DR.3 Decreased tear film function is exhibited in a decreased TBUT, as well as Schirmer’s test values, increased corneal vital dye staining, and increased signs of conjunctival metaplasia.3,9
NK is a disease of the cornea resulting from trigeminal nerve damage, which ODs know occurs with diabetes. Many of the early signs and symptoms of NK are similar to DED. One can lead to the other, and are related to the loss of corneal homeostasis. The main differentiation is reduced or absent corneal sensation in NK.
There are three stages of NK, as defined by the Mackie classification: Stage 1 exhibits epithelial changes only, such as irregularity of the epithelium, punctate keratopathy, and corneal edema, with reduced or absent sensations in one or more quadrants of the cornea. Stage 2 is loss of corneal sensation, along with a recurrent or persistent epithelial defect without stromal involvement. Finally, Stage 3 is the aforementioned findings of Stages 1 and 2, along with stromal involvement, leading to a corneal ulcer, melting and, ultimately, perforation.
IN THE TREATMENT PIPELINE
The following NK treatments are in the pipeline:
CSB-001. (Claris Biotherapeutics, Inc.). See https://bit.ly/3wLZpZJ .REC 0/0559. (Recordati Rare Diseases) See https://bit.ly/3j9WIsV .
OC-01 Nasal Spray. (Oyster Point Pharma, Inc.) See https://bit.ly/3DX1B2p .
TREATMENT
In Ms. Jones’ case, and in similar cases, optometrists need to first provide patient education on how blood sugar levels are related to ocular dryness, in addition to DR. From there, ODs must convey the importance of better blood sugar control. Optometrists should make sure to communicate with the patient’s primary care physician and, possibly, suggest the patient see a dietician for guidance.
Next, ODs should treat any DED signs aggressively, regardless of the patient’s symptoms, due to the corneal effects discussed above. Sometimes, getting the patient on board with the treatment plan can be challenging, which is why patient education regarding the link between diabetes and DED is so important.
In Ms. Jones’ case, I prescribed multiple therapies at once: in-duction therapy with topical fluorometholone 0.1% b.i.d. OU to help calm the inflammation; cyclosporine ophthalmic solution b.i.d. OU, an omega fatty acid supplement; heat mask with lid massage; and lid hygiene. Depending on the patient’s DED presentation, optometrists may want to start at a different rung on the treatment ladder and move up based on patient response. (For additional DED treatments, see “Heal, Clean, Calm and Protect the Ocular Surface,” at https://bit.ly/3uCbkXC .)
With respect to NK, treatment is similar. By taking the previous steps, ODs may be able to help prevent progression to NK. Use of amniotic membranes have proven effective in studies, as have autologous serum tears.6,10 In some cases, these treatments are combined with a surgical intervention, such as tarsorrhaphy, or in the case of corneal perforation, corneal transplants may be necessary.
Cenegermin-bkbj (Oxervate, Dompé), which uses a recombinant nerve growth factor (NGF), has proven successful in the clinical trial for treatment of both Stages 2 and 3 of NK.10,11,12 NGF is important because it helps maintain corneal integrity by cell proliferation, tear secretion, and corneal reinnervation. By reintroducing it to the tear film, it helps to stimulate these pathways and resolve NK. Patients use the drop q2h, six times a day, for eight weeks. Retreatment may be necessary in more aggressive cases of NK, though longevity of treatment has only been studied out so far. Further, patients who have underlying chronic systemic causes of NK, such as diabetes, may have reoccurrences and need retreatment as well. (See “In the Treatment Pipeline,” p. 24.)
FRONT AND CENTER
The case of Ms. Jones serves as an important reminder that patients who have diabetes are at a high risk for both DR and corneal involvement. Thus, optometrists should perform a thorough slit lamp exam with vital dyes even when the patient does not complain of symptoms related to DED. ODs must also be proactive in treating these patients prior to any signs or symptoms, knowing that these patients are statistically more likely to, at some point, have corneal diabetic damage. Finally, when DED clinical signs reveal themselves, and/or patients report DED symptoms, the optometrist should treat more aggressively to decrease the risk of progression. OM
REFERENCES
- Age-1. Semeraro F, Cancarini A, dell’Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res. 2015;2015:582060. doi: 10.1155/2015/582060
- Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190-194. doi:10.4103/ijo.IJO_646_17
- Nepp J, Abela C, Polzer I, Derbolav A, Wedrich A. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea. 2000;19(4):487-91. doi: 10.1097/00003226-200007000-00017.
- Zhao H, He Y, Ren YR, Chen BH. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol. 2019;12(12):1939-1950. doi: 10.18240/ijo.2019.12.17.
- Zhang, X, Zhao L, Deng S, Sun X, Wang N., et al. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol. 2016;2016:8201053. doi: 10.1155/2016/8201053.
- Matsumoto, Y, Dogru M, Goto E., et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004; 111(6):1115-1120. doi: 10.1016/j.ophtha.2003.10.019.
- Ruiz-Lozano, Raul E., Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A. The molecular basis of neurotrophic keratopathy: diagnostic and therapeutic implications. A review. The Ocular Surface. 19 (2021): 224-240. doi: 10.1016/j.ophtha.2019.08.020.
- Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77(11):554–558. doi: 10.1016/j.optm.2006.08.002.
- Zhmud, TM, Drozhzhyna GI,d Demchuk AV. Cytological features of the bulbar conjunctiva in patients with type 2 diabetes mellitus. J Ophthalmol. (Ukraine) 2021;1(498) 24-28.
- Sacchetti, M., Komaiha, C., Bruscolini, A., et al. Long-term clinical outcome and satisfaction survey in patients with neurotrophic keratopathy after treatment with cenegermin eye drops or amniotic membrane transplantation. Graefes Arch Clin Exp Ophthalmol. 2022;260(3):917-925. doi: 10.1007/s00417-021-05431-6.
- Bonini S, Lambiase A, Rama P et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125(9):1332-1343. doi: 10.1016/j.ophtha.2018.02.022.
- Pflugfelder SC, Massaro-Giordano M, Perez VL et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology. 2020;127(1):14-26.




