A fluid approach to glaucoma care
After appropriate testing, a 50-year-old patient was diagnosed as a high-risk glaucoma suspect OD, and with early glaucoma OS. To get a better of idea of his potential peak IOP after only a few visits, the Water Drinking Test (WDT) was performed. The WDT is accomplished after a 2-hour liquid fast by obtaining baseline IOP readings, followed by IOP readings at 15, 30, and 45 minutes after drinking 800 ml (27 ounces) to 1 L of water within 5 minutes.1**
Here’s a look at why the WDT may be important to you and your patients, and how to implement its investigational use into clinical practice.
WHY IS THE WDT IMPORTANT?
The WDT has been proposed as a sort of stress test to assess outflow facility. Specifically, and in no particular order, various studies have suggested that the WDT:
- Strongly correlates with diurnal tension curve (DTC)2 and successfully predicts DTC peak IOP.3
- May be a surrogate that indirectly reflects trabecular and/or uveoscleral outflow facility.2,3
- Confirms the effectiveness, patency, and superiority of trabeculectomy compared to medically treated patients due to less WDT response among trabeculectomy patients.4,5
- Aids in identifying patients who have normal tension glaucoma and are most at risk for VF progression.6,7
- Has shown relatively more of a response in females younger than age 50, among patients who have higher IOP at baseline and those who use a greater number of medications.3
- Helps determine the effectiveness of topical medical therapy, with a lower-to-higher IOP spike among patients taking latanoprost, brimonidine, and timolol, respectively, and a slower return to baseline in the latter group.3,1*
- Demonstrates lower IOP spikes in surgically treated patients, with quicker return to the normal range compared to medically treated patients.1
- Identifies IOP instability and determines how the eye responds to transient IOP spikes.1
- Results may be a risk factor (6 times increased risk) for developing glaucoma if the WDT response exceeds 5 mmHg compared with those who have a WDT response less than 1 mmHg among glaucoma suspect patients.8
- Provides excellent IOP diurnal tension curve (DTC) reproducibility and reliability,9,10 even among pseudoexfoliation syndrome and pseudoexfoliation glaucoma patients.11
- Elevated IOP response may be secondary to a combination of increased plasma hypo-osmolarity-induced aqueous ultrafiltration, stimulation of the autonomic nervous system, elevated episcleral venous pressure, and/or choroidal expansion.1,12
- May determine other informative factors such as “…the IOP elevation (peak IOP – baseline IOP), the time to peak IOP and time to recovery to baseline IOP.”1***
- Shows peak IOP earlier (within 10 to 15 minutes) with a quicker return to baseline IOP among normal patients12 (suggesting higher-functioning outflow facility1), and delayed peak IOP levels (30 to 45 minutes) with a slower return to the normal range among glaucoma and ocular hypertension patients.13
- IOP elevation may be more significant among angle-closure patients than open-angle glaucoma patients due to observed choroidal thickening.14
- Aids in better detecting those patients who are outside the target IOP range and are, therefore, showing disease progression despite normal, stable in-office IOP measurements.15
- Is associated with the stage of the glaucoma severity.16****
- May be a stress test for retinal ganglion cell function, as suggested by significantly delayed pattern electrogram latency.17
- May cause angle closure in susceptible patients18,19 but is not a reliable test to determine which patients need prophylactic iridotomies.19
- May be a useful, provocative test for the detection of IOP peaks in both primary open-angle glaucoma and primary angle-closure glaucoma patients.20
- Shows both reduced IOP fluctuations and peak IOP levels among glaucoma and ocular hypertension patients following selective laser trabeculoplasty (SLT).21
- Does not diagnose glaucoma due to low sensitivity and low specificity.22,1,9
- Is not considered to have “positive” or “negative” test results.1
- Is contraindicated in patients who have coexisting systemic diseases, (cardiac, renal, or history of urinary retention).2
HOW CAN THE WDT HELP?
IOP is a dynamic balance among four factors: (1) aqueous production, (2) trabecular meshwork outflow, (3) uveoscleral outflow, and (4) episcleral venous pressure. These are also dynamic and continually changing23,24 based on many variables, including patient positioning, medication use, age, and time of day, to name just a few.
The fluctuating endpoint from this clinical quartet helps explain fluctuating IOPs throughout a 24-hour period.25 As these values are the basis for our clinical decision making to initiate treatment or escalate therapy (topical, laser, surgical) to prevent the progression and/or development of glaucoma,26-32 we need a data set that is both accurate and extensive. Because IOP levels are measured “…only rarely and mostly randomly, perhaps a few times a year in most patients…”33, and because a majority of patients potentially have their highest IOP readings outside normal clinic hours,34 the WDT is a brief, inexpensive chairside approach that may provide actionable and representative information in addition to DTC1 studies and or home-monitoring/sleep studies.
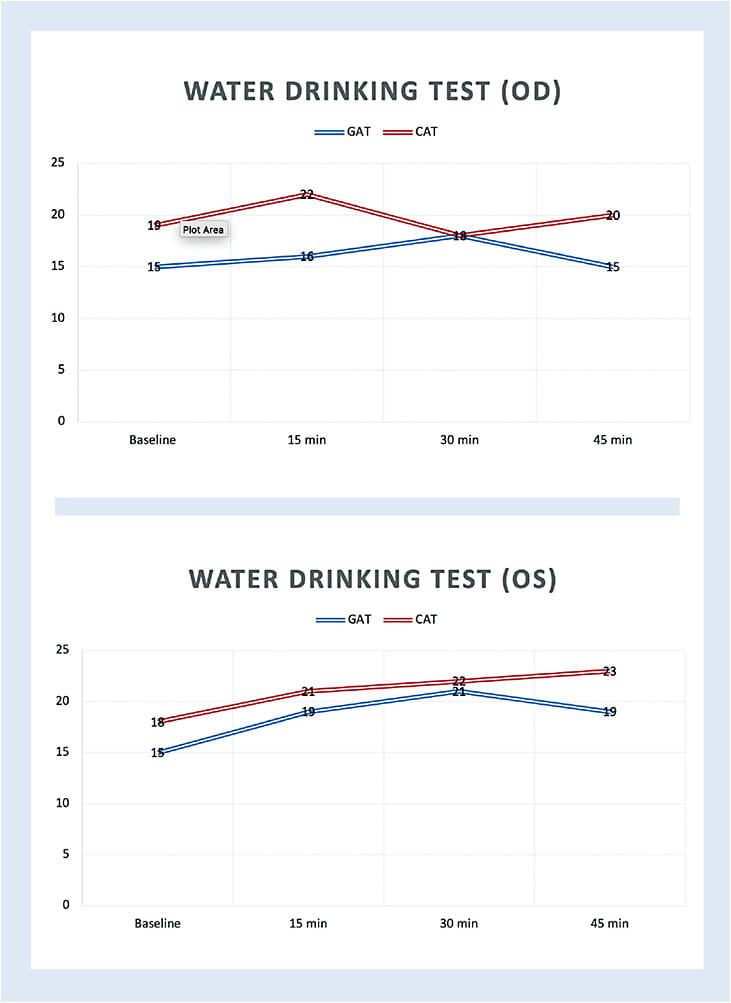
DISTILLED DATA...
Based on the graph for this patient (above), note that the glaucomatous left eye had a greater IOP elevation, slower time to peak IOP, and a slow/incomplete return to baseline (within the allotted time frame) IOP levels compared to the fellow, glaucoma-suspect right eye. As a result of this additional, representative information, the patient was scheduled for SLT OS only, with plans to repeat the WDT a couple months postoperatively. OM
References:
1. Vasconcelos-Moraes CG, Susanna R Jr. Correlation between the water drinking test and modified diurnal tension curve in untreated glaucomatous eyes. Clinics. 2008 Aug;63(4):433-6. doi: 10.1590/s1807-59322008000400004.
2. Razeghinejad MR, Tajbakhsh Z, Nowroozzadeh MH, Havens SJ, Ghate D, Gulati V. The Water-Drinking Test Revisited: An Analysis of Test Results in Subjects with Glaucoma. Semin Ophthalmol. 2018;33(4):517-524. doi: 10.1080/08820538.2017.1324039.
3. Chen CH, Lu DW, Chang CJ, Chiang CH, Chou PI. The application of water drinking test on the evaluation of trabeculectomy patency. J Ocul Pharmacol Ther. 2000;16(1):37-42. doi: 10.1089/jop.2000.16.37.
4. Danesh-Meyer HV, Papchenko T, Tan YW, Gamble GD. Medically controlled glaucoma patients show greater increase in intraocular pressure than surgically controlled patients with the water drinking test. Ophthalmology. 2008;115(9): 1566-70. doi: 10.1016/j.ophtha.2008.01.023.
5. Yoshikawa K, Inoue T, Inoue Y. Normal tension glaucoma: the value of predictive tests. Acta Ophthalmol (Copenh). 1993;71(4): 463-70. doi: 10.1111/j.1755-3768.1993.tb04619.x.
6. Susanna R Jr, Vessani RM, Sakata L, Zacarias LC, Hatanaka M. The relation between intraocular pressure peak in the water drinking test and visual field progression in glaucoma. Br J Ophthalmol. 2005;89(10): 1298-1301. doi: 10.1136/bjo.2005.070649.
7. Susanna R Jr, Clement C, Goldberg I, Hatanaka M. Applications of the water drinking test in glaucoma management. Clin Exp Ophthalmol. 2017;45(6): 625-631. doi: 10.1111/ceo.12925.
*”This may be an alternative explanation for the results of LoGTS, in which, despite its limitations, especially regarding the highest dropout rate in the brimonidine group, similar IOP (single measurements) were obtained in both groups, whereas the rate of visual field progression was higher in the timolol group.”
**“It is not yet clear whether the use of 1000 mL, 800 mL or 10 mL/kg bodyweight improves the correlation or predictive value of the WDT.”
***”Usually but not always, eyes with higher IOP peaks after water ingestion take longer to return to baseline IOP levels than eyes with lower IOP peaks, which may reflect the status of the drainage system of the eye.”
8. Armaly MF, Krueger DE, Maunder L, et al. Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual-field defects. Arch Ophthalmol. 1980;98(12):2163-71. doi: 10.1001/archopht.1980.01020041015002.
9. Landers J. Challenging glaucoma with a water-drinking test. Clin Exp Ophthalmol. 2015;43(3):200-1. doi: 10.1111/ceo.12503.
10. Babic, M., De Moraes, C., Hatanaka, M., Ju, G., Susanna, R. Reproducibility of the water drinking test in treated glaucomatous patients. Clin Exp Ophthalmol. 2015 Apr;43(3):228-33.
doi: 10.1111/ceo.12434.
11. Özyol, E., Özyol, P. and Karalezli, A. (2016), Reproducibility of the water-drinking test in patients with exfoliation syndrome and exfoliative glaucoma. Acta Ophthalmol. 2016;94(8): e795-e798. doi: 10.1111/aos.13132.
12. Ulaş F, Balbaba M, Celebi S. Effects of a water-loading test on intraocular pressure and corneal hysteresis in young healthy subjects. J Glaucoma. 2014;23(2):101-4. doi: 10.1097/IJG.0b013e318264ce7c.
13. Hatanaka M, Alencar LM, De Moraes CG, Susanna R Jr. Reproducibility of intraocular pressure peak and fluctuation of the water-drinking test. Clin Exp Ophthalmol. 2013;41(4): 355-9. doi: 10.1111/j.1442-9071.2012.02882.x.
14. Arora KS, Jefferys JL, Maul EA, Quigley HA. Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci. 2012 Sep 21;53(10):6393-402. doi: 10.1167/iovs.12-10224.
15. Malerbi FK, Hatanaka M, Vessani RM, Susanna R Jr. Intraocular pressure variability in patients who reached target intraocular pressure. Br J Ophthalmol. 2005;89(5):540-2. doi: 10.1136/bjo.2004.058230.
16. Susanna CN, Susanna BN, Susanna FN, Susanna R Jr, De Moraes CG. Peak Intraocular Pressure Time during Water Drinking Test and Its Relationship with Glaucoma Severity. J Ophthalmic Vis Res. 2022 21;17(1):27-32. doi: 10.18502/jovr.v17i1.10167.
**** “We found that the time during WDT of IOP peaks' occurrence was associated with glaucoma severity in a population with treated POAG. Specifically, eyes with more severe disease had a later IOP peak than eyes with less severe disease.”
17. Gameiro G, Monsalve P, Golubev I, Ventura L, Porciatti V. Neurovascular Changes Associated With the Water Drinking Test. J Glaucoma. 2018;27(5): 429-432. doi: 10.1097/IJG.0000000000000898.
18. Venugopal N. Water drinking test and angle closure glaucoma. Indian J Ophthalmol. 2015;63(2):172. doi: 10.4103/0301-4738.154410.
19. Arora KS, Jefferys JL, Maul EA, Quigley HA. Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci. 2012;53(10):6393-6402. doi:10.1167/iovs.12-10224
20. Poon YC, Teng MC, Lin PW, Tsai JC, Lai IC. Intraocular pressure fluctuation after water drinking test in primary angle-closure glaucoma and primary open-angle glaucoma. Indian J Ophthalmol. 2016;64(12): 919-923. doi: 10.4103/0301-4738.198851.
21. Kerr NM, Lew HR, Skalicky SE. Selective Laser Trabeculoplasty Reduces Intraocular Pressure Peak in Response to the Water Drinking Test. J Glaucoma. 2016;25(9): 727-31. doi: 10.1097/IJG.0000000000000512.
22. Roth JA. Inadequate diagnostic value of the water-drinking test. Br J Ophthalmol. 1974;58(1):55-61. doi:10.1136/bjo.58.1.55
23. Brubaker RF. Flow of Aqueous humor in humans. [The Friedenwald Lecture] Invest Ophthalmol Vis Sci. 1991;32(13):3145-66.
24. Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24: 612-637. doi: 10.1016/j.preteyeres.2004.10.003.
25. Liu JH, Kripke DF, Twa MD, Hoffman RE, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912-7.
26. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6): 701–830. doi: 10.1001/archopht.120.6.701.
27. Miglior S, Zeyen T., Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005. 112(3): 366-375. doi: 10.1016/j.ophtha.2004.11.030.
28. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–79. doi: 10.1001/archopht.120.10.1268.
29. Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118(9): 1766–73. doi: 10.1016/j.ophtha.2011.01.047.
30. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4): 429–40. doi: 10.1016/s0002-9394(00)00538-9.
31. Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090-5. doi: 10.1001/archopht.1991.01080080050026.
32. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126: 487-97. doi: 10.1016/s0002-9394(98)00223-2.
33. Weinbreb R.N., Brandt J.D., Garway-Heath D., et al. World Glaucoma Association Consensus Series 4 – Intraocular Pressure. Kugler Publications, The Hague, The Netherlands. 2007.
34. Liu JHK, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586-90. doi: 10.1167/iovs.02-0666.




