Dry eye disease (DED) affects our patients’ vision and quality of life. Like many diseases, early diagnosis is key in managing patients. Early diagnosis means early intervention and better results for both the patient and practitioner. In advanced stages, DED can be more difficult to manage: Comorbidities, such as allergy, can complicate the outcomes, and symptoms don’t always equate to a simple clinical presentation. I believe it’s essential to screen every patient for DED (using a questionnaire), evaluate the cornea with vital dye, and push on the meibomian glands to ascertain functionality. Since DED is chronic and progressive, patients may receive the DED diagnosis at varying stages. (See “DED Prevention,” p.24.)
Here, I discuss how I stage DED patients and the treatments I prescribe as a result, with a full list of their benefits, as they appear under each stage. These treatments are based on the diagnostic algorithm from the International Dry Eye Workshop II.1,2 (See “Additional Treatments,” p.25.)
A caveat: Treatment should also be based on the patient’s overall health, specific symptoms, and underlying causes.3

Stage 1 DED
Diagnostic criteria. These patients present with a standard patient evaluation of eye dryness (SPEED)* score of less than 6 (asymptomatic), 5/5 meibomian gland expression in all three quadrants, cloudy gland secretion, and less than 25% of gland loss on meibography. Additionally, their lid margins reveal minimal debris, no collarettes, no telangiectasia, no corneal surface staining, no conjunctival surface staining, nor injection.
Treatment. Given these findings, I would prescribe lid hygiene,* an ocular nutritional supplement, artificial tear drops, and a follow-up appointment at six months. Additionally, for additional no-cost early lifestyle changes, the provider could recommend blinking exercises/awareness, a low-inflammatory diet (e.g., less-processed foods), and good hydration.
* For a full list of DED questionnaires, visit bit.ly/DEDDiagnosticSurveys .
* Lid hygiene should include debulking collarettes — pathognomonic for Demodex — with lid cleanser, hypochlorous spray for possible Staphylococcus aureus, the 20/20/20 rule (bit.ly/202020RULEAOA ), home heat therapy, and best practices for cosmetics and beauty habits.
Stage 2 DED
Diagnostic criteria. I classify Stage 2 DED as a SPEED score of 6, foreign body sensation, photophobia, ocular itching, episodic or activity-based visual symptoms, 3-4/5 meibomian gland expression in all quadrants, semi-solid opaque gland secretion, and 26% to 50% gland loss or truncation on meibography. Further, Stage 2 DED patients can have mild telangiectasia, trace blepharitis, trace to 2+ staining (non-central), trace 2+ staining on the conjunctival surface, though no injection, and tear break-up time (TBUT)/osmolarity/MMP-9 <10sec/>308/(+).4
Treatment. Based on these findings, I would prescribe Stage 1 DED treatment, along with moisture goggles and ointment (the latter if the patient’s symptoms are worse in the morning and the patient has poor lid seal or lagophthalmos), an immunomodulator (see new option in “Leading Off,”), neurostimulation (chemical or mechanical), and a corticosteroid qid for two weeks, then bid for two weeks. Additionally, I would prescribe in-office-heat and meibomian gland expression, in-office lid debridement, and follow-up at one month. If at follow-up, the patient presents with late-Stage 2 DED, I would insert punctal plugs after controlling DED-related inflammation.
DED PREVENTION
Prevention of DED can be achieved by instructing patients to employ these habits:
- Proper blinking and practicing the 20/20/20 rule.
- Lid hygiene.
- Removing toxic ingredients from everyday routines.
- Practicing safe eye beauty habits.
- Drinking water.
- Taking ocular nutritional supplements.
Stage 3 DED
Diagnostic criteria. These patients present with a SPEED score greater than 6, eye watering, ocular pain, chronic visual symptoms (e.g., blurriness) that limit their activities, 1 to 2 meibomian gland expression in all quadrants, solid opaque gland expression, and 51% to 75% gland loss or truncation on meibography. Further, they have moderate lid margin telangiectasia with mild-to-moderate blepharitis, 3 to 4+ diffuse superficial punctate keratitis, or corneal surface central staining, hyperemia, moderate staining of the conjunctival surface, and TBUT/osmolarity/MMP-9- <5 sec / > 308 / (+). (Incidentally, at this point, I would consider corneal sensitivity testing to check for a neurotrophic cornea if the patient wasn’t symptomatic.)
Treatment. Given these findings, I would prescribe an amniotic membrane, an antibiotic eye drop used according to the prescribed dosage, intense pulsed light (IPL) therapy (one treatment every two-to-four weeks for four to five treatments), followed by heat and meibomian gland expression in office. Additionally, I would prescribe a subsequent maintenance treatment at three to six months, along with heat and meibomian gland expression, and autologous serum up to eight times a day.
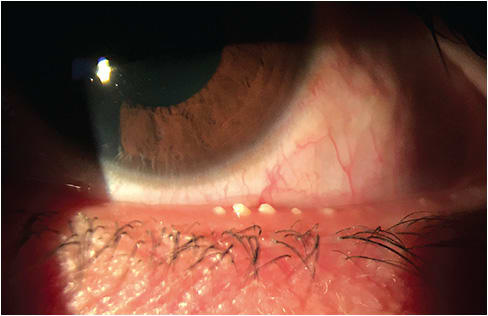
Stage 4 DED
Diagnostic criteria. I classify Stage 4 DED as severe symptoms (i.e., disabling, decrease in BCVA, constant ocular irritation or watering, inability to perform daily tasks), lack of meibomian gland expression in any or all quadrants, and 75% to 100% gland loss on meibography. Further, Stage 4 DED patients have severe lid margin telangiectasia and blepharitis, severe corneal staining, neurotrophic keratitis, conjunctival hyperemia, severe conjunctival staining, and TBUT/osmolarity/MMP-9- immediate / >308 / (+). Or worse, they have no symptoms at all, with Stage 2 to Stage 3 neurotrophic keratitis (NK) and possible NK ulcers.
Treatment. Based on these findings, I would prescribe cenegermin, amniotic membranes (typically five-to-seven days), neurostimulation up to 10 times a day with mechanical neurostimulation, autologous serum six to eight times per day, and scleral contact lenses. This regimen will depend on how the patient responds. Modifications can be made once the patient is stable. Also, I would consider conducting in-house meibomian gland probing, punctal cautery, tarsorrhaphy, and referral for surgical interventions, if the patient is at risk of permanent vision loss.5
ADDITIONAL TREATMENTS
- Bandage contact lens. This can reduce inflammation, stabilize the ocular surface and tear film, and improve corneal healing.10
- Biologic platelet-rich plasma (PRP) drops. DED signs and symptoms can be decreased after treatment with such drops, as they reduce the immunologic component.11
- Low level light therapy (LLLT). LLLT is a device that works through photobiomodulation. It can be utilized on any skin type and is provided in the office for patients. Many doctors utilize it alongside IPL. LLLT is comprised of applying red, or near infra-red, radiation employing low-power light sources to relieve pain, decrease inflammation, and promote tissue repair.12
- Ophthalmic inserts. These stabilize and thicken the tear film, prolonging TBUT, while lubricating and protecting the cornea.”13
- Oral tetracycline analogues. These result in small to major reductions in the abnormal appearance of the glands (from -4% to -89%) and increase in tear film stability (from 21% to 273%).14
- Perfluorohexyloctane ophthalmic solution. This forms a monolayer at the air-liquid interface of the tear film, which can be expected to decrease evaporation.15
- Topical testosterone. Androgens, such as testosterone, have been reported to restore the reduced lipid phase of the tear film.16
Benefits of mentioned treatments
- Lid hygiene. This clears debris, over invasive bacteria and Demodex, helping to maintain homeostasis.
- Ocular nutritional supplement. Omega-3 and omega-6 fatty acids contain anti-inflammatory properties.6
- Artificial tears. Preservative-free formulations are generally prescribed to preclude corneal toxicity, which can occur due to the long-term use of preservative-containing drops.
- Warming moisture goggles. These are shown to temporarily relieve ocular discomfort and improve tear film stability via creating “a humid ‘microclimate’ maintained by cutaneous transpiration.”7,8
- Ointment. This treatment provides moisture to the cornea in cases of severe corneal exposure, as is the case with lagophthalmos. It is typically applied to the cornea at bedtime, as this is when the eye remains closed for the longest period.
- Immunomodulator. Cyclosporine blocks T-cell activation and is a calcineurin inhibitor, consequently inhibiting inflammatory cytokine production (selective inhibition of IL-I). Additionally, cyclosporine treatment has been shown to increase goblet cell density in the conjunctiva.
- Lymphocyte function-associated antigen-1 (LFA-1) antagonist. Lifitegrast blocks the binding between LFA-1 and ICAM-1, which results in the inhibition of T-cell migration into target tissues. This, in turn, reduces cytokine release and decreases further T-cell recruitment.
- Neurostimulation. This results in endogenous tear production, or a patient’s real tears, giving patients a way to manage their DED, and achieve immediate relief.
- In-office heat with meibomian gland expression. Heat or thermal treatment helps unblock the meibomian glands. This unblocking aids in resuming the natural production of meibum (oil) needed for a stable tear film.
- Lid margin debridement. The procedure removes the accumulated bacterial biofilm from the lid margin and scurf from the eyelashes.9) This enables the delivery of meibum to the lid margin.
- Corticosteroid. Topical corticosteroids quell the ocular inflammation associated with DED, which leads to symptomatic relief. That said, it should only be used typically two weeks to four weeks, as it can increase IOP.
- Punctal plugs. These allow tears to stay in the eye longer vs. draining through the canaliculus into the nasolacrimal system. When considering plugs, optometrists should make sure the inflammation has been treated first, as not treating it creates more inflammatory factors present on the front surface of the eye, which can exacerbate DED symptoms.
- Amniotic membrane. Available in cryopreserved or dehydrated form, amniotic membranes act as therapeutic bandages, restoring ocular surface health.
- IPL treatment. This shrinks or destroys the small vessels that contribute to the inflammation associated with DED and ocular rosacea.
- Autologous serum. This treatment contains growth factors and nutrients found in healthy tears. (I usually start with 40%, but they can be reconstituted from 20% up to 60%.) Local and national labs obtain these for patients.
- Cenegermin. This 0.002% topical solution contains a recombinant form of human nerve growth factor, a protein made by the human body that acts through specific high-affinity and low-affinity nerve growth factor receptors in the anterior segment of the eye. This supports corneal innervation and integrity. It is prescribed for patients who have NK.
- Scleral contact lens. Scleral contact lenses contain a sterile water bath that can support the front surface of an eye that has DED, and any corneal irregularities.
- Meibomian gland probing. Using a 2 mm probe, this treatment eliminates gland scar tissue, so the glands can deliver meibum (oil) to create tears.
- Punctal cautery. This treatment permanently closes the tear duct, so tears remain on the ocular surface.
Set the stage
To increase the likelihood of successful DED treatment, it’s important to keep DED on your diagnostic radar and be ready to intervene early. Understanding the range of treatment options available and tailoring them to the severity of the condition allow for effective management and improved outcomes. Regular follow-up appointments are essential to monitor treatment effectiveness and make any necessary adjustments. My normal follow-up is four weeks after treatment induction, and I never let my patients go longer than six months. OM
References
- Sullivan, DA, Rocha, EM, Aragona, P, et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul Surf. 2017;15(3):284-333. doi: 10.1016/j.jtos.2017.04.001. doi: 10.1016/j.jtos.2017.05.004.
- Craig JP, Nichols K, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283. doi: 10.1016/j.jtos.2017.05.008.
- Rolando, M, and Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45. doi: 10.1016/s0039-6257(00)00203-4.
- Schiffman, RM, Walt, JG, Jacobsen, G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412-9. doi: 10.1016/S0161-6420(03)00462-7.
- Yu, J, Asche CV, Fairchild, CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 30(4), 379-387. doi: 10.1097/ICO.0b013e3181f7f363.
- Barabino S, Horwath-Winter J, Messmer EM, Rolando M, Aragona P, Kinoshita S. The role of systemic and topical fatty acids for dry eye treatment. Prog Retin Eye Res. 2017;61:23-34. doi: 10.1016/j.preteyeres.
- Yueping R, Chen J, Zheng Q, Chen W. Short-term effect of a developed warming moist chamber goggle for video display terminal-associated dry eye. BMC Ophthalmol. 2018;18(1):33. doi: 10.1186/s12886-018-0700-y.
- Leopold P. Very useful eyeglasses. J Fr Ophtalmol. 1980;3(12):759-62.
- Moon SY, Han SA, Kwon HJ, et al. Effects of lid debris debridement combined with meibomian gland expression on the ocular surface MMP-9 levels and clinical outcomes in moderate and severe meibomian gland dysfunction. BMC Ophthalmol. 2021;21(1):175. doi: 10.1186/s12886-021-01926-2.
- Wu X, Ma Y, Chen X, et al. Efficacy of bandage contact lens for the management of dry eye disease after cataract surgery. Int Ophthalmol. 2021;41(4):1403-1413. doi: 10.1007/s10792-021-01692-6.
- Sanchez-Avila RM, Merayo-Lloves J, Riestra AC, et al. The Effect of Immunologically Safe Plasma Rich in Growth Factor Eye Drops in Patients with Sjögren Syndrome. J Ocul Pharmacol Ther. 2017;33(5):391-399. doi: 10.1089/jop.2016.0166.
- Markoulli M, Chandramohan N, Papas EB. Photobiomodulation (low-level light therapy) and dry eye disease. Clin Exp Optom. 2021;104(5):561-566. doi: 10.1080/08164622.2021.1878866.
- Nguyen T, Latkany R. Review of hydroxypropyl cellulose ophthalmic inserts for treatment of dry eye. Clin Ophthalmol. 2011; 5: 587–591. doi: 10.2147/OPTH.S13889
- Doughty MJ. On the prescribing of oral doxycycline or minocycline by UK optometrists as part of management of chronic Meibomian Gland Dysfunction (MGD). Cont Lens Anterior Eye. 2016;39(1):2-8. doi: 10.1016/j.clae.2015.08.002.
- Miebo. Highlights of Prescribing Information. https://www.bausch.com/globalassets/pdf/packageinserts/pharma/miebo-package-insert.pdf . (Accessed June 12, 2023.)
- Worda C. Nepp J, Huber JC, Sator MO. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas. 2001 Jan 31;37(3):209-12. doi: 10.1016/s0378-5122(00)00181-x.




