Sharing my clinical experience using low-dose atropine on patients
Topical atropine has been widely used to slow myopia progression, with concentrations ranging from 0.01% to as high as 1% in some East Asian countries. Most of the randomized clinical trials (RCTs) report the higher the concentration administered, the better the myopia-controlling efficacy.1,2,3,4,5 Additionally, most of the studies report minimal side effects, such as light sensitivity and near blur, as well as great tolerability in trial cohorts.1,2,3,4,5 As a result, the use of low-dose atropine (LDA) has shifted toward a higher concentration at treatment initiation.
Despite the superior tolerability of LDA reported in the clinical trials, I would like to discuss some real-world clinical experiences that may not have been seen or reported in the studies. These observations were made at my clinic, the UC Berkley Myopia Control Clinic in Berkley, Calif., in approximately 60 patients receiving LDA treatments seen during more than 200 office visits.
PUPIL CHANGES
It has been reported that static pupil size increases with atropine concentration, with an average increase of 1.25 mm, 0.67 mm, and 0.60 mm in 0.05%, 0.025%, and 0.01% atropine groups, respectively.3 As a result, many practitioners are using static pupil measurement to monitor for treatment compliance or to predict the severity of light sensitivity.
However, in my clinical experience, the pupil size, and the change of it in children using LDA treatment varies dramatically depending on the time of the day of the measurement, the technique of the measurement, and the pharmacy of choice.
There is also significant individual variability in pupil dilation induced by LDA, for which some patients who used a 0.025% concentration had smaller magnitude of dilation than those in 0.01%. Additionally, the static pupil size measurement has such huge fluctuation, that the range of pupil size is more meaningful than a random measure (Image).
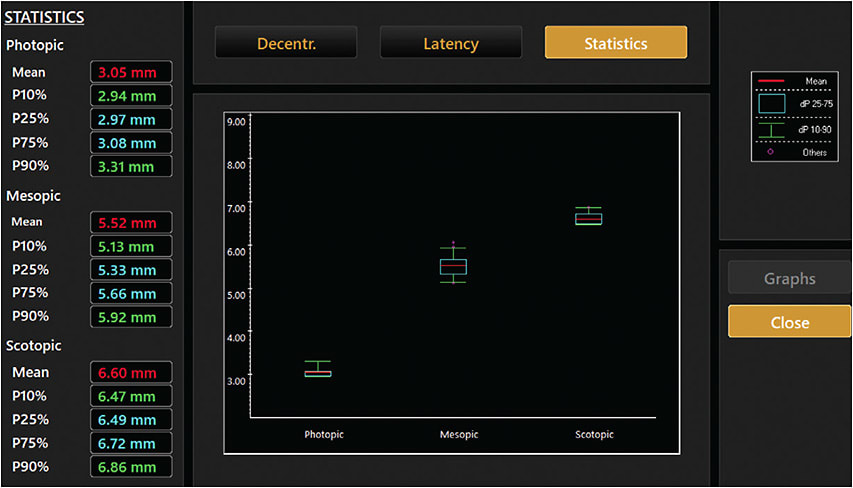
Due to the huge individual variability in pupil dilation after LDA treatment, as well as the within-subject measurement variability, a professional pupillometer that offers consecutive measurements of pupil sizes under good control of ambient lighting and accommodation is a more suitable choice to monitor pupil changes under LDA treatment. Additionally, the range of the pupil size should be recorded than a random reading to better depict the overall pupil size and its micro-fluctuation.
LDA CONCENTRATION AND PHOTOPHOBIA
In my clinical experience, 0.01% atropine causes little photophobia and near blur in most of the patient population I have treated, regardless of iris color.
Around 10% to 25% of patients under 0.02% to 0.025% atropine treatment showed some level of photophobia.
Interestingly, how the symptoms were “solicited” made a significant difference. For example, when a child was asked “do you notice any sensitivity to light,” or “do you have any difficulty playing outdoor due to bright light,” the answers were usually negative.
However, when the parents were asked “how often does your child need to wear sunglasses when playing outdoors?” often times the answer was “all the time.” It was very clear that any side effects or symptoms-related questions need to be addressed in a more specific, “leading question,” such as the one above, and it should be assumed that some side effects, like photophobia, are present until clearly proven otherwise by the patient or their parents’ answers.
Subjective questionnaires designed for clinical studies may not reliably reflect either the frequency or the severity of the side effects. More than 40% of the children using 0.05% atropine showed some level of light sensitivity, which is much more than what was reported in clinical trials.1,2,3 Despite good compliance to the treatment, many children were not happy due to the side effects and eager to be switched to a lower concentration or a different treatment.
Another interesting finding in my practice is that pupil size poorly predicts the severity of photophobia, for which many children with minimal dilation showed moderate-to-significant photophobia and some with dramatic dilation but very little subjective complaints. Preliminary studies are ongoing to investigate whether the velocity and the amplitude of pupil constriction in response to light better predict the severity of symptoms.
WHAT TO KEEP IN MIND ABOUT LDA
The use of LDA for myopia control is well supported with RCTs, however the careful decisions on the initial concentration and the close monitoring of the patients’ symptoms and ocular signs are critical to ensure the long-term safety of the treatment and patient satisfaction. In particular, the frequency and severity of light sensitivity with LDA treatments in real-world practice tend to be higher than what was reported in RCTs. OM
REFERENCES
- Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study. Ophthalmology. (2019) 126:113–24. doi: 10.1016/j.ophtha.2018.05.029,
- Yam JC, Li FF, Zhang X, Tang SM, Yip BHK, Kam KW, et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Ophthalmology. (2020) 127:910–9. doi: 10.1016/j.ophtha.2019.12.011,
- Yam JC, Zhang XJ, Zhang Y, Wang YM, Tang SM, Li FF, et al. Three-year clinical trial of low-concentration atropine for myopia progression (LAMP) study: continued versus washout: phase 3 report. Ophthalmology. (2022) 129:308–21. doi: 10.1016/j.ophtha.2021.10.002
- Hieda O, Hiraoka T, Fujikado T, Ishiko S, Hasebe S, Torii H, et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. (2021) 65:315–25. doi: 10.1007/s10384-021-00822-y.
- Saxena R, Dhiman R, Gupta V, Kumar P, Matalia J, Roy L, et al. Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology. (2021) 128:1367–9. doi: 10.1016/j.ophtha.2021.01.026




