The recent FDA approvals of the first two therapies for geographic atrophy (GA) make a strong argument that the diagnosis and monitoring of this condition are more important than ever. (See “GA: An Overview,” p.29) The structural imaging of retinal abnormalities and lesion growth by imaging tools is essential for diagnosing and monitoring progression to GA.1 The tools used for this endeavor are confocal near-infrared reflectance, fluorescein angiography, fundus autofluorescence, fundus photography, and SD-OCT.
SD-OCT provides cross-sectional 3D visualization of different retinal layers and the choroid to aid the eye care provider in localizing and identifying the clinical features associated with GA.2
Here, we discuss the features of GA that can be revealed by SD-OCT.
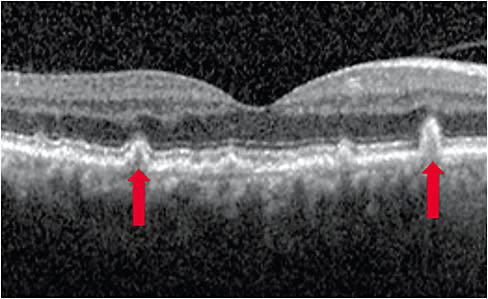
Reticular pseudodrusen
SD-OCT can show a drusen subtype known as reticular pseudodrusen (RPD), which has been associated with greater progression to GA.3-7 Depending on the stage of RPD development, it can appear as hyperreflective intraretinal deposits: between the retinal pigment epithelium (RPE) breaking through the ellipsoid zone layer and appearing as cones in Stage 3 age-related macular degeneration (AMD), and fading within the inner retinal layers in Stage 4 AMD.3,4,8
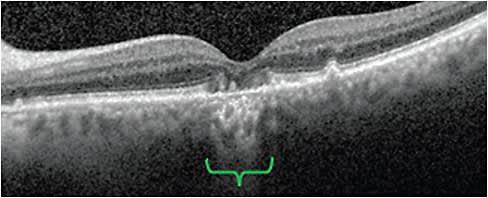
Hyperreflective foci
Hyperreflective foci viewed through SD-OCT are associated with increased disease progression.9-12 Hyperreflective foci appear as dot-like lesions that typically lack back shadowing, do not have a representative visible fundus lesion, and whose reflectivity is below 30 µm.12
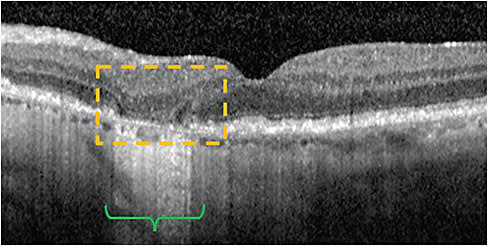
Hypertransmission defects
Also known as hyperTDs, these are visible as bright regions, resulting from increased light passing through the choroid. This light passing is due to photoreceptor and outer retinal degradation, which are characteristic of later stages of AMD.13
HyperTDs have been used by the Classification of Atrophy Meeting retinal experts to define atrophic evolution based on SD-OCT, including the following: complete RPE and outer retinal atrophy (or cRORA), incomplete RPE and outer retinal atrophy (or iRORA), complete outer retinal atrophy (or cORA), and incomplete outer retinal atrophy (or iORA).14 GA is considered a subset of cRORA, though it is characterized by more extensive features: 1) HyperTD regions of ≥250 µm in diameter; 2) an area of RPE attenuation or disruption ≥250 µm in diameter; and 3) photoreceptor degradation.14
Nascent GA
Also referred to as nGA, this is considered a subset of iRORA, which precedes cRORA and is characterized by: 1) hyperTD regions; 2) corresponding RPE attenuation or disruption with or without persistent basal laminar deposits; and 3) photoreceptor degeneration.14,15,16
Wu et al (2020) prospectively evaluated nGA as a predictor for developing GA in 284 eyes from 142 participants. Of the 12 eyes that developed color fundus photograph-defined GA, 10 demonstrated SD-OCT-defined nGA.16
Other clinical features
SD-OCT can also be used to view clinical features associated with increased GA progression once patients are diagnosed at this late stage. These include lesions that are extrafoveal (nonfoveal), larger in size, multifocal, and presenting bilaterally.18,17,20
GA: AN OVERVIEW
GA is characterized by sharp atrophic retinal lesions resulting from the progressive degeneration of photoreceptors, the retinal pigment epithelium (RPE), and the choriocapillaris residing beneath the RPE.19 As a result, over time it can result in irreversible vision loss.19
Patients with newly diagnosed GA may initially have spared central vision, particularly if the GA lesion is outside the fovea. While deficits in visual acuity measurements may not be observed until the GA lesion encroaches the fovea, patients may report challenges with functional vision, such as difficulty with reading or recognizing faces, and impaired constrast sensitivity.28
The first two therapies for GA are pegcetacoplin injection, (Syfovre, Apellis), FDA-approved in Feb. 2023 (https://syfovreecp.com ), and avacincaptad pegol intravitreal solution (Izervay, Iveric Bio, an Astellas Company), FDA-approved in Aug. 2023. (izervay.com ).
It’s all about image
Advances in OCT technology and further use and investigations by the eye care and research community, along with multimodal imaging use, may help continue to enhance the utility of OCT technology for AMD and GA patients.14,2,21 Such advances include wider fields of view, greater resolution, and greater accessibility for both patients and their health care providers, including the creation of handheld, at-home, and intraoperative OCT devices.22 Furthermore, artificial intelligence, machine learning, and deep-learning programs for the diagnosis, management, and prediction of GA are expected to play significant roles in increasing the accuracy, precision, and capabilities of OCT, enabling optimal care for patients.23-27
Medical writing support was provided by IMPRINT Science, New York, NY, and was funded by Iveric Bio, an Astellas Company. OM
References
- Geographic Atrophy: American Academy of Ophthalmology EyeWiki. https://eyewiki.org/Geographic_Atrophy . Accessed August 17, 2023.
- Holz FG, Sadda SR, Staurenghi G, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124(4):464-478. doi: 10.1016/j.ophtha.2016.12.002.
- Khan KN, Mahroo OA, Khan RS, et al. Differentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Progress in retinal and eye research. 2016;53:70-106. doi: 10.1016/j.preteyeres.2016.04.008
- Querques G, Canouï-Poitrine F, Coscas F, et al. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(3):1264-1270. doi: 10.1167/iovs.11-9063.
- Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619-1625. doi: 10.1016/j.ophtha.2011.01.029.
- Joachim N, Mitchell P, Kifley A, et al. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology. 2013;120(10):2042-2050. doi: 10.1016/j.ophtha.2013.03.029
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BEK. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145(2):317-326. doi: 10.1016/j.ajo.2007.09.008.
- Sivaprasad S, Bird A, Nitiahpapand R, et al. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016;61(5):521-537. doi: 10.1016/j.survophthal.2016.02.005.
- Ho J, Witkin AJ, Liu J, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118(4):687-693.
- Folgar FA, Chow JH, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53(8):4626-4633.
- Nassisi M, Fan W, Shi Y, et al. Quantity of intraretinal hyperreflective foci in patients with intermediate age-related macular degeneration correlates with 1-year progression. Invest Ophthalmol Vis Sci. 2018;59(8):3431-3439.
- American Academy of Ophthalmology. Hyperreflective Foci in Optical Coherence Tomography. https://eyewiki.aao.org/Hyperreflective_Foci_in_Optical_Coherence_Tomography (Accessed August 28, 2023)
- Liu J, Laiginhas R, Corvi F, et al. Diagnosing Persistent Hypertransmission Defects on En Face OCT Imaging of Age-Related Macular Degeneration. Ophthalmol Retina. 2022;6(5):387-397. 10.1016/j.oret.2022.01.011.
- Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration: classification of atrophy meeting report 4. Ophthalmology. 2020;127(3):394-409. doi: doi: 10.1016/j.ophtha.2019.09.035.
- Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028.
- Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121(12):2415-2422. doi: 10.1016/j.ophtha.2014.06.034.
- Wu Z, Luu CD, Hodgson LAB, et al. Prospective Longitudinal Evaluation of Nascent Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmol Retina. 2020;4(6):568-575. doi: 10.1016/j.oret.2019.12.011.
- Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271-277.
- Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390. doi: 10.1016/j.ophtha.2017.08.038.
- Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural History of Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123(2):361-368. doi: 10.1016/j.ophtha.2015.09.036.
- Ong J, Zarnegar A, Corradetti G, Singh SR, Chhablani J. Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J Clin Med. 2022;11(17): (17):5139. doi: 10.3390/jcm11175139.
- Kapoor R, Whigham BT, Al-Aswad LA. Artificial Intelligence and Optical Coherence Tomography Imaging. Asia-Pacific J Ophthalmol. 2019;8(2):187-194. doi: 10.22608/APO.201904.
- Dahrouj M, Miller JB. Artificial Intelligence (AI) and Retinal Optical Coherence Tomography (OCT). Semin. Ophthalmol. 2021;36(4):341-345. doi: 10.1080/08820538.2021.1901123.
- Mai J, Lachinov D, Riedl S, et al. Clinical validation for automated geographic atrophy monitoring on OCT under complement inhibitory treatment. Scientific Rep. 2023;13(1):7028. doi: 10.1038/s41598-023-34139-2.
- Vogl WD, Riedl S, Mai J, et al. Predicting Topographic Disease Progression and Treatment Response of Pegcetacoplan in Geographic Atrophy Quantified by Deep Learning. Ophthalmol Retina. 2022;7(1):4-13. doi: 10.1016/j.oret.2022.08.003.
- Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Invest Ophthalmol Vis Sci. 2018;59(8):3199-3208. doi: 10.1167/iovs.18-24106.
- Anegondi N, Gao SS, Steffen V, et al. Deep Learning to Predict Geographic Atrophy Area and Growth Rate from Multimodal Imaging. Ophthalmol Retina. 2023. ;7(3):243-252. doi: 10.1016/j.oret.2022.08.018.
- Bakri SJ, Bektas M, Sharp D, Luo R, Sarda SP, Khan S. Geographic atrophy: Mechanism of disease, pathophysiology, and role of the complement system. J Manag Care Spec Pharm. 2023;29(5-a Suppl):S2-S11). doi: 10.18553/jmcp.2023.29.5-a.s2.





