This article was originally published in a sponsored newsletter.
The past year was marked by innovation and rapid advances in the macular disease space. With the recent dissemination of clinical trial and other research data, we are already seeing changes in the ways we practice.
Advanced imaging systems continue to provide valuable information. Fundus autofluorescence (FAF) has come into its own as a way to characterize the metabolic status of the retinal pigment epithelium (RPE). OCT and OCT-angiography (OCT-A) change how clinicians diagnose and manage AMD and other retinal diseases. Along with en-face OCT, infrared (IR) and near-IR imaging have proven useful in tracking progression vs. stability in geographic atrophy (GA).
Newly approved pharmacotherapies have revolutionized care for many of our patients who live with nAMD. Today’s retina specialists choose from an increasing array of anti-VEGF and other medication options. Delivered by intravitreal injection, faricimab (Vabysmo®) received its initial U.S. FDA approval for nAMD and diabetic macular edema (DME) in 2022.1 In 2023, its application was expanded to include treatment for macular edema secondary to retinal vein occlusion.2 This agent goes beyond standard anti-VEGF therapy to target both the VEGF and non-VEGF signaling pathways. Faricimab is a bispecific antibody that binds to and inhibits both VEGF-A and angiopoietin-2.1
Aflibercept 8 mg (Eylea HD®) has also received FDA approval for the treatment of nAMD, DME and diabetic retinopathy. This approval was based on two multicenter, double-masked, active-controlled, pivotal Phase 3 clinical trials: PULSAR for nAMD and PHOTON for DME.3 The recommended dose for this agent is 8 mg (0.07 mL of 114.3 mg/mL solution) administered via intravitreal injection every four weeks (monthly) for the first three months, followed by 8 mg every eight to 16 weeks.3
Biosimilar drugs—a type of biologic medicine that is highly similar to an FDA-approved drug—also continued to emerge. FYB201 (CIMERLI®) is a biosimilar to ranibizumab (Lucentis®). The COLUMBUS-AMD clinical trial validated the safety and efficacy of FYB-201 for the treatment of nAMD-associated macular edema in a multicenter, evaluation-masked, parallel-group, 48-week, Phase 3 randomized study.4
On the non-neovascular AMD side, pegcetacoplan (SYFOVRE®), a complement C3 inhibitor, was approved in February 2023 as the first medication to treat geographic atrophy (GA).5 It is administered through monthly and bimonthly injections. Clinicians have long been aware that the alternative complement pathway plays a critical role in the progression of GA.
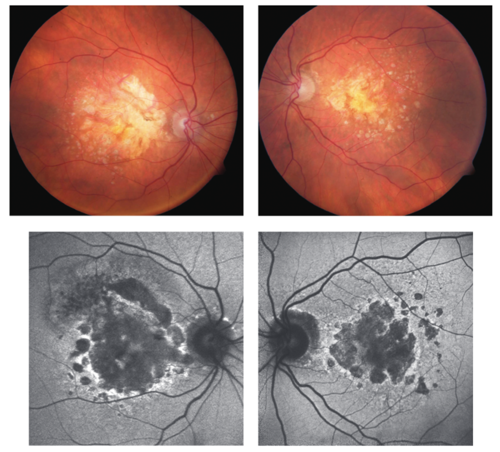
A second treatment, avacincaptad pegol (IZERVAY®), was also FDA approved for GA in 2023. This agent is a complement C5 inhibitor with a similar dosing method and schedule to pegcetacoplan.6 Both pegcetacoplan and avacincaptad pegol have been shown to slow the progression and growth rate of atrophic lesions (see Figure 1). We’ll review some of the clinical trial and real-world efficacy and safety data on both these drugs in coming issues.
We look forward to bringing you another informative year of AMD Clinical Insights.
Key Clinical Takeaways
- The past year has seen many advances in retina and AMD research.
- Imaging technologies continue to improve and provide valuable information for clinical decision-making.
- An increasing array of anti-VEGF and other medication options are available for nAMD, including two new agents.
- Both faricimab and aflibercept 8 mg reduce the nAMD treatment burden with longer times between injections.
- Two medications were approved to treat GA: pegcetacoplan and avacincaptad pegol. While they do not stop the disease, they do reduce the rate of progression.
Reference(s):
- Genentech. New Vabysmo data suggest greater retinal drying versus aflibercept in wet age-related macular degeneration and diabetic macular edema. April 25, 2023. Accessed January 8, 2024. https://www.gene.com/media/press-releases/14990/2023-04-25/new-vabysmo-data-suggest-greater-retinal
- Genentech. FDA approves Genentech’s Vabysmo for the treatment of retinal vein occlusion (RVO). October 26, 2023. Accessed January 8, 2024. https://www.gene.com/media/press-releases/15009/2023-10-26/fda-approves-genentechs-vabysmo-for-the-
- GlobalData Healthcare. AAO 2023: PULSAR and PHOTON studies provide promising results for AMD and DME. Pharmaceutical Technology. November 7, 2023. Accessed January 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/aao-2023-pulsar-photon-studies-promising-results-amd-dme/
- Holz FG, Oleksy P, Ricci F, et al. Efficacy and safety of biosimilar FYB201 compared with ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2022 Jan;129(1):54-63. doi:10.1016/j.ophtha.2021.04.031.
- Apellis. FDA approved SYFOVRETM (pegcetacoplan injection) as the first and only treatment for geographic atrophy (GA), a leading cause of blindness. February 17, 2023. Accessed January 8, 2024. https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only
- Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021 Apr;128(4):576-586. doi:10.1016/j.ophtha.2020.08.027




