As we have all seen in our careers, patients often have multiple diseases when they present for an evaluation. Some of these diseases may be systemic, and we need to be aware of the implications of those diseases on the patient’s eye health. Other times, those additional diseases may in fact be ophthalmic diseases that can cloud the clinical picture of the disease process that we are monitoring. A good example of this is in the case below.
This particular patient has a long-standing history of primary open-angle glaucoma (POAG) that I have been treating for many years. As with patients of increasing age, comorbid diseases can play a role in making it more difficult to monitor their glaucoma. It so happens that along with this patient’s history of hypertension and hyperlipidemia, he also developed a small branch retinal artery occlusion (BRAO). We know the effects of acute retinal artery occlusions can cause abrupt change in the patient’s vision, which is often the reason they present to the office on an urgent basis. But also keep in mind the structural aftermath of changes that occur in these eyes with loss of retinal tissue diffusely in the area supplied by the particular artery.
One of the many advantages of the Spectralis OCT in the management of glaucoma is the GMPE software, which facilitates looking at and analyzing several different anatomic areas that are affected by glaucoma. With this glaucoma software, several different size diameter retinal nerve fiber layer (RNFL) scans can be evaluated, as well as the neuroretinal rim itself, as measured by the Bruch's membrane opening minimum rim width (BMO-MRW) scans. Furthermore, there is ample evidence that glaucomatous damage can be seen in the macular ganglion cell layer. For these reasons, when I am evaluating a patient with glaucoma, I will always look at all three areas closely. I’m looking for subtle deterioration, especially since this can occur in only one or two areas scanned. Significant progressive structural damage will eventually manifest as changes in all three areas, but subtle disease progression may occur only in one area.
In Figure 1, we see the macular ganglion cell scan of this patient, taken 6 months after the initial BRAO. Note in the scan that the relative paucity of the ganglion cell layer (GCL) thickness secondary to the infarct. In fact, you can clearly see where the BRAO caused damage; this was a macular twig artery occlusion, originating from the superior arcade and extending inferiorly to the temporal fovea. While prior to the BRAO we would have been able to use the macular GCL scans to monitor for progression of the glaucoma, now that we are 6 months post BRAO event, we’ve lost some of the ability to use the GCL scan in this eye to monitor for worsening glaucoma. You can, though, continue to use the GCL format in this eye to monitor for progression of the glaucoma in the inferior hemisphere, but in reality, we have lost about 50% of the usefulness of this scan due to the artery occlusion.
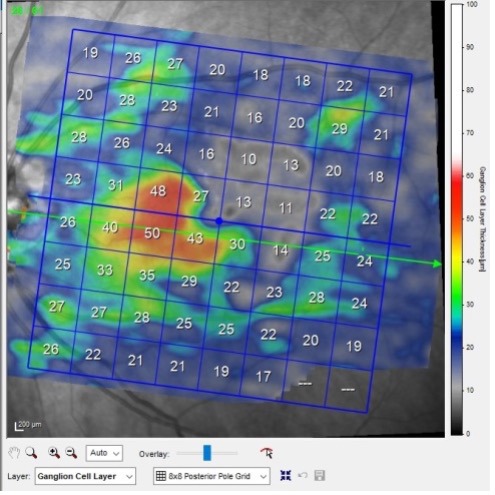
In Figure 2, you can readily see the loss of the retinal tissue temporal to the fovea in the selected B scan, secondary to the artery occlusion.
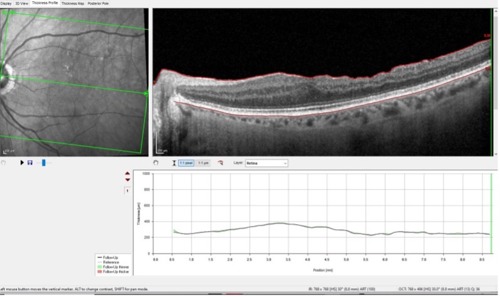
So, if we have a confounding event affecting our ability to evaluate the GCL in this eye, what tools do we have left to ascertain stability or progression of glaucoma? This is where the Glaucoma Module Premium Edition software comes in, where we can specifically evaluate several different circumpapillary RNFL scans and evaluate the neuroretinal rim itself for changes over time.
Notice in Figure 3 that we can evaluate the 3.5 mm diameter RNFL circle scan for glaucomatous damage. Also in Figure 3, there are two other larger diameter circle scans that can be evaluated, though I would be cautious using the largest 4.7 mm scan simply because that scan is far enough away from the optic nerve that it may show damage from the artery occlusion as well.
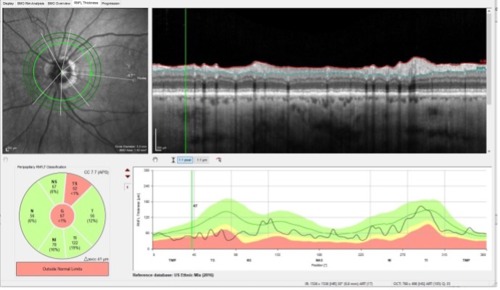
Close examination of Figure 3 demonstrates loss of RNFL tissue superior temporally, which is where this patient actually had glaucomatous damage prior to the artery occlusion; now that same sector has also suffered damage from the BRAO—all the more reason why dissecting out the details in this area will be difficult to assess. Are the changes from glaucoma, from the artery occlusion, or both?
Where, then, is the area furthest away from the artery occlusion we can look to evaluate for glaucomatous damage without having the artery occlusion confound the data? The answer here is simply at the optic nerve head. Again, the GMPE software comes in handy, as seen in Figure 4.
In Figure 4, we are looking at the actual neuroretinal rim of this patient. While artery occlusions have profound structural changes in all tissues supplied by the occluded artery, the offending artery was the macular twig variety, which provided little if any blood supply to the neuroretinal rim. And herein lies our main focus of future evaluations for progressive glaucomatous damage, namely the neuroretinal rim itself.
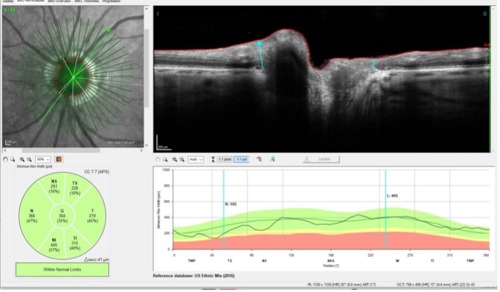
Having the ability to see the big picture when it comes to glaucoma and being able to evaluate all of the areas that can show progressive glaucomatous damage, especially in cases like this where there are comorbid processes that can mimic glaucoma, is clinically invaluable. It affords us the opportunity to provide better patient care and assure ourselves that our diagnoses are valid.
This editorial content was supported via unrestricted sponsorship




