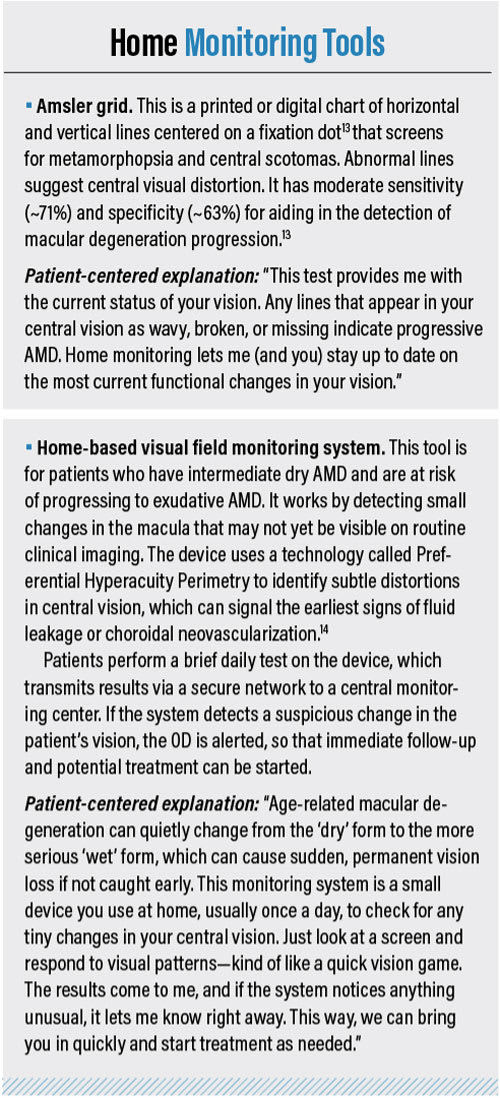
Optometrists must be aware of the applications of the latest age-related macular degeneration (AMD) diagnostics for 3 main reasons: (1) to diagnose the condition accurately and early, (2) to use the findings to inform patients on interventions and the importance of regular follow-up visits, and (3) to monitor AMD for disease progression. What’s more, patients tend to be loyal to optometrists who provide the most up-to-date technology.
This article discusses the value of the latest diagnostic tools (in alphabetic order) and provides related patient-centered explanations, so patients become motivated to be active participants in their care (see “Home-Monitoring Tools,” below).
Adaptive Optics Ophthalmoscopy
This deformable mirror or wavefront sensor captures retinal images at the cellular level to compensate for optical aberrations.1 Specifically, it enables the visualization of individual photoreceptors and retinal pigment epithelial (RPE) cells, aiding in the early detection of AMD.
Patient-centered explanation: “This instrument lets me see any changes to your retinal cells at the cellular level before they cause tissue damage. It’s noninvasive and takes just a few minutes per eye. The earlier we catch any changes, the better our chances of preserving your vision.”
Artificial Intelligence-Assisted Management of AMD
Artificial intelligence (AI) tools are becoming increasingly prevalent in the detection and management of AMD. These systems use machine-learning algorithms trained on thousands of retinal images to identify subtle signs of disease. AI can detect drusen, pigmentary changes, geographic atrophy (GA), and even early neovascularization with high sensitivity from imaging results. Some platforms offer real-time risk stratification, progression tracking, or predictive modeling to help clinicians make faster and more accurate decisions.
Beyond diagnosis, AI is also being integrated into home-monitoring tools, thereby offering more consistent and accessible care, especially in underserved areas and busy clinics.
Patient-centered explanation: “Technology is making big advances in how we care for eyes, and AI is one of the most exciting tools we now have in the fight against AMD. AI stands for artificial intelligence, and in simple terms, it means we’re using computer programs that have learned how to ‘read’ eye scans. These systems can detect very early signs of AMD and track even the smallest changes over time. One useful feature of AI is that it allows us to feel confident that nothing gets missed, especially when we’re looking at very detailed scans. It also helps us figure out which patients need treatment sooner, and which ones can be safely monitored. In the future, AI may even let patients monitor their eyes from home using smart devices, and help doctors give you fast and accurate care.”
Contrast Sensitivity Testing
Contrast sensitivity (CS) measurement, available via the Pelli-Robson chart or a validated electronic CS chart, reveals the visual system’s response to the wide range of light and levels of contrast associated with daily activities.2 This is important because research demonstrates CS is connected to real-world visual function, including identifying faces, signs, and objects.3
Patient-centered explanation: “This test aids me in identifying any problems with your ability to discern contrast. It’s important I assess this, as AMD can affect one’s contrast prior to their clarity. Contrast sensitivity is an important part of functional vision, especially for daily activities like driving, so CS testing is another way to help protect all aspects of your vision.”
Dark Adaptometry
Dark adaptometry measures the retina’s recovery of visual sensitivity in darkness after bright light ex-posure.4 Impaired dark adaptation is an early functional biomarker of AMD, often preceding observable structural changes in the retina.⁴ Studies show that patients with AMD, even in its earliest stages, exhibit significantly delayed rod-mediated dark adaptation compared to age-matched controls.⁴ Incidentally, devices that test macular optical density, which is associated with dark adaptation, are available.
Patient-centered explanation: “An important way that we assess your retina is by checking how well your eyes adjust from bright light to darkness. The retina’s ability to adapt to light changes is an indication of how well it’s working. In people who have early macular changes, this adjustment can take longer than average.
We can use a simple, noninvasive test that measures how your eyes recover in the dark after being exposed to light. This test helps us detect changes earlier and track them over time, which means we can manage your eye health more effectively and potentially slow disease progression.”
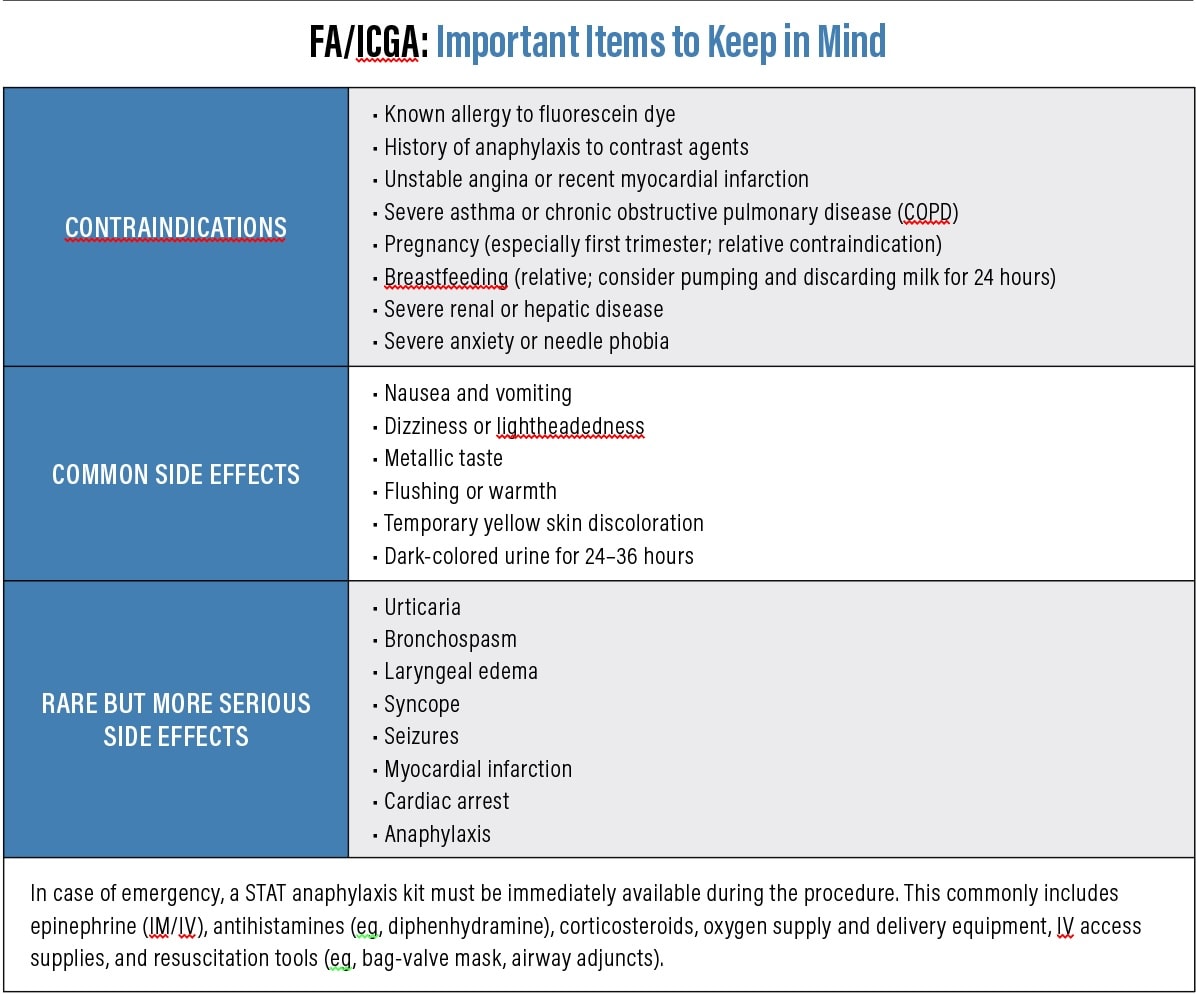
Fluorescein Angiography
Fluorescein angiography (FA) is used to detect and characterize choroidal neovascularization (CNV) in neovascular AMD.5 The intravenous injection of fluorescein dye and sequential imaging enables the OD to identify retinal leakage, staining, and pooling.
Patient-centered explanation: “This test helps me see how your blood flows to your retina, and whether all the areas of the retina are getting as much blood as they should, or if there are areas of new growth of blood vessels.”
Genetic Testing for AMD
These tests usually screen for mutations in genes like the complement cascade on chromosome 1 and ARMS2/HTRA genes on chromosome 10.6 Genetic testing can provide valuable information about disease risk, expected progression, and, in some cases, treatment responsiveness.
Although genetic testing is not routinely recommended for AMD patients at this time, it is being increasingly explored for early detection in high-risk individuals (eg, those with a strong family history) and in clinical trial selection for emerging gene-targeted therapies.
Patient-centered explanation: “AMD can run in families, and part of the reason is that some people are born with certain changes in their DNA that make them more likely to develop this condition over time. Genetic testing is comprised of a simple cheek swab or blood test that helps us look for these specific changes. While having a certain gene doesn’t mean you’re guaranteed to get AMD, it gives us an idea of your personal risk and can tell us whether you’re more likely to develop the type of AMD that worsens quickly. This information doesn’t change your vision today, but it helps us tailor your care. For example, we might monitor you more closely, offer advice about lifestyle changes (See the online article, “Educating Patients on AMD Modifiable Risk Factors”) or consider you for new therapies or clinical trials based on your results.”
Indocyanine Green Angiography
Indocyanine green angiography (ICGA) uses a dye to help image blood circulation in the retina and choroid with a focus on the choroidal structures. It aids in the detection of choroidal neovascularization, particularly in distinguishing classic and occult CNV, and can be used with FA to detect leakage and end-capillary perfusion.7
Patient-centered explanation: “This test involves injecting yellow or green dye into your arm. The dye travels to the blood vessels in your eye, enabling detailed pictures of any abnormal or leaking blood vessels in your retina. The dye is safe, and your skin or urine may appear yellow for a few hours (see sidebar: “FA/ICGA: Important Items to Keep in Mind,” below).
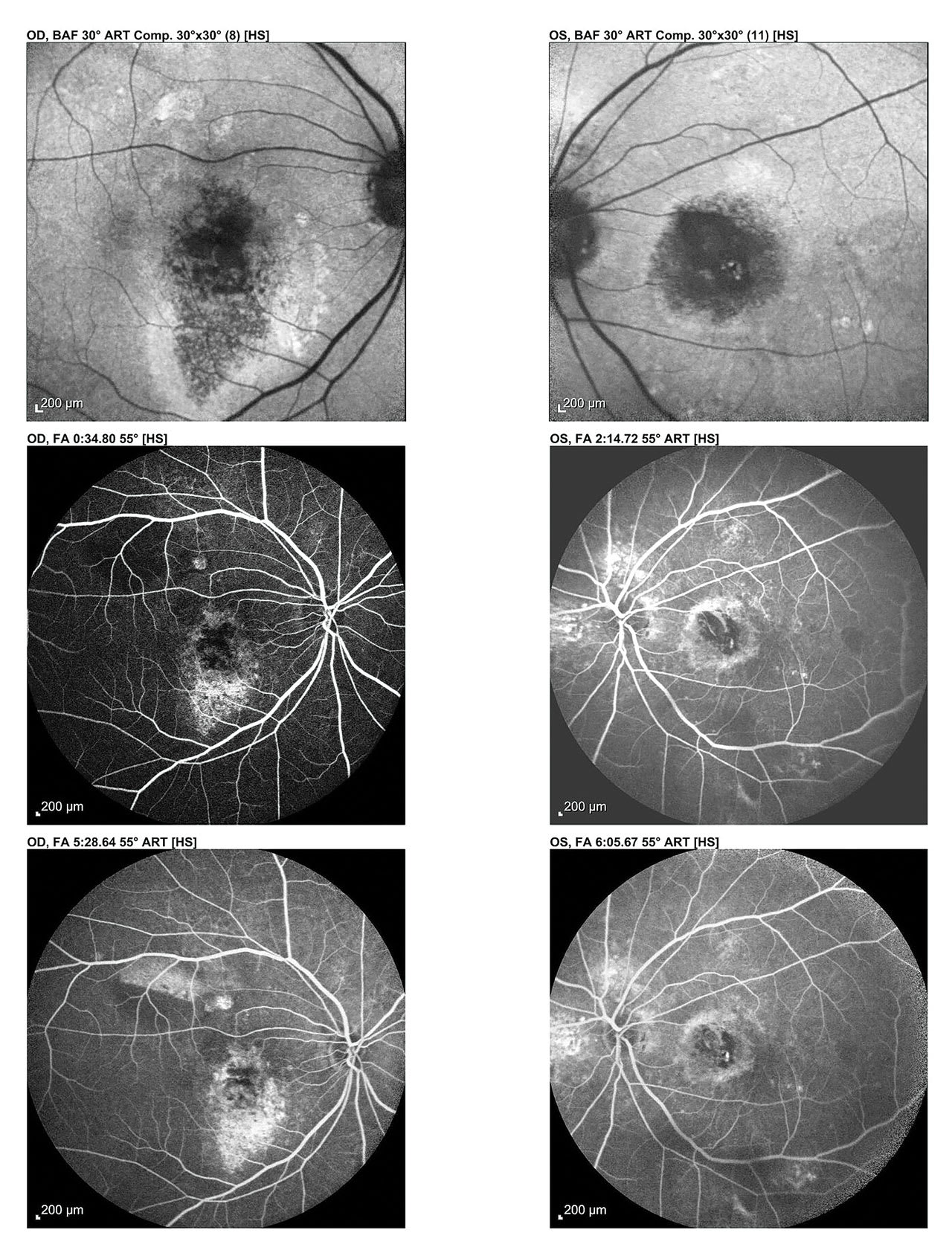
Fundus Autofluorescence (FAF)
This uses specific light wavelengths to cause natural retinal pigments in the RPE to fluoresce.8 Areas of increased fluorescence indicate stress. Dark areas indicate RPE atrophy. FAF is particularly helpful for detecting GA and in providing patient education: patients can often intuitively understand these images.
Patient-centered explanation: “This scan uses light to highlight areas of stress or damage in the retina’s pigment layer before they appear in regular photos. Bright areas might indicate too much waste build-up, and dark areas may indicate retinal damage.”
Fundus Photography
This captures high-resolution color images of the retina, macula, optic nerve, and blood vessels, enabling the documentation of drusen, pigment changes, hemorrhages, and GA. Also, comparisons of serial photographs aid in the detection of lesion progression. Like FAF images, fundus photography can be a useful patient-education tool.
Patient-centered explanation: “This image details the back of your eye, helping me to identify any signs of AMD. It also shows changes in retina color and pigment—important signs of disease progression.” Microperimetry This procedure tests retinal sensitivity at discrete points in the central visual field while tracking fixation.9 Specifically, it maps areas of reduced or absent visual responses, correlating structural imaging with functional loss. Essentially, microperimetry provides objective metrics that play a role in detecting subtle changes before patients experience vision decline.
Patient-centered explanation: “The macula is the part of the eye that’s responsible for the most sensitive and detailed vision. When someone has AMD, some of their ability to notice these specific details starts to deteriorate. This instrument can measure the sensitivity of your retina and help me see how much vision remains. This way, we can talk about how the disease might impact your lifestyle, and we can make treatment decisions.”
Multifocal Electroretinography
Multifocal electroretinography (ERG) is a test that measures the electrical activity of many small areas of the retina by using a photostress test to identify functional disturbances, even in the absence of morphologic signs. Multifocal ERG uses a corneal or bulbar conjunctival electrode to record local electrophysiological responses. Specifically, delays in multifocal ERG responses indicate that the relative intensity of photoreceptors inner segment ellipsoids is reduced, which is an early feature of AMD.10
Patient-centered explanation: “Your retina works by producing tiny electrical signals when light reaches the back of your eye. These signals are then transmitted to your brain via the optic nerve, and your brain interprets this electrical activity as vision. Small electrodes are placed near your eyeball and you’ll be asked to look at a screen that has flickering patterns flashing in different spots. Just look at the screen, and the machine will interpret how your eyes are deciphering the patterns. AMD often causes poor electrical signaling of the retinal cells, so this test has value throughout the progression of the disease to let me know whether the management plan should be changed.”
Optical Coherence Tomography
Optical coherence tomography (OCT) is a noninvasive imaging method that provides high-resolution, cross-sectional views of the retina. In dry AMD, it aids the OD in identifying drusen, RPE irregularities, and GA. In wet AMD, it helps the optometrist detect subretinal and intraretinal fluid, pigment epithelial detachments, and cystoid macular edema. OCT findings also inform responses to anti-VEGF therapy.11 There are 2 major types of OCT instruments: spectral-domain OCT (SD-OCT) and swept-source OCT (SS-OCT). SD-OCT machines provide excellent imaging of the retinal layers. SS-OCTs penetrate deeper and image the choroid and choriocapillaris. Both types can be used to gauge disease progression.
Patient-centered explanation: “This is a quick, painless scan that uses light waves to obtain de- tailed pictures of your retina. This technology actually “looks behind” the eye to aid me in assessing your retina’s health. This scan will be repeated once or several times a year, depending on any noted changes, such as fluid or swelling, to your retina.”
Optical Coherence Tomography Angiography
OCT angiography (OCTA) images retinal and choroidal microvasculature without dye injection.5 Specifically, the technology aids in detecting motion contrast from blood cells to visualize CNV networks (types 1, 2, and 3), maps vascular structures, and is convenient for AMD monitoring.
Patient-centered explanation: “This scan shows tiny blood vessels in your retina without any dye. It takes a few minutes, is noninvasive, and helps me detect abnormal vessel growth or changes. This scan gives me an idea of areas that could leak and cause problems in the future. This technology will help me to predict serious complications, such as vision loss from fluid or bleeding in your retina.”
10–2 Visual Field Testing
The 10–2 visual field (VF) test measures sensitivity at 68 points within the central 10° of the macula using a computerized perimeter.12 Specifically, it aids in the detection of small central scotomas. Results include threshold sensitivity, mean deviation, and pattern deviation. It is reproducible and valuable for tracking functional loss.
Patient-centered explanation: “Measuring your central VF helps me get an idea of how well you’re seeing the visual space in front of you. The results help me detect even minor blind spots and monitor changes over time.”
A Winning Combination
Each diagnostic technology provides unique insights into the structural and physiologic status of the retina, enabling early detection, accurate staging, and the ongoing monitoring of AMD progression and/or treatment response. Equally important is the ability of clinicians (and/or allied health staff—alter patient explanations as needed) to explain these tools in clear, relatable terms that empower patients to participate actively in their care. By combining clinical precision with empathetic education, ODs can improve patient adherence, detect changes sooner, and ultimately preserve vision and quality of life for patients. OM
References
1. Burns SA, Elsner AE, Sapoznik KA, Warner RL, Gast TJ. Adaptive optics imaging of the human retina. Prog Retin Eye Res. 2019;68:1-30. doi:10.1016/j.preteyeres.2018.08.002
2. Roark MW, Stringham JM. Visual performance in the ‘real world’: contrast sensitivity, visual acuity, and effects of macular carotenoids. Mol Nutr Food Res. 2019; 63: e1801053. 2019;63(15):1801053)
3. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br J Ophthalmol. 1987;71:791-6.
4. Nigalye AK, Hess K, Pundlik SJ, Jeffrey BG, Cukras CA, Husain D. Dark Adaptation and Its Role in Age-Related Macular Degeneration. J Clin Med. 2022;11(5):1358. Published 2022 Mar 1. doi:10.3390/jcm11051358.
5. Mahapatra SK, Mohanty A, Bidasaria A, Parhi A. Comparison of swept source – optical coherence tomography angiography with fundus fluorescein angiography for detection of lesions in diabetic retinopathy. Taiwan Journal of Ophthalmology. Published online May 2, 2025. doi:10.4103/tjo.tjo-d-24-00117
6. Trincão-Marques J, Ayton LN, Hickey DG, et al. Gene and cell therapy for age-related Macular degeneration: A review. Survey of Ophthalmology. 2024;69(5):665-676. doi:10.1016/j.survophthal.2024.05.002
7. Herbort CP Jr, Mantovani A, Tugal-Tutkun I, Papasavvas I. Classification of Non-Infectious and/or Immune Mediated Choroiditis: A Brief Overview of the Essentials. Diagnostics (Basel). 2021;11(6):939. Published 2021 May 24. doi:10.3390/diagnostics11060939
8. Kellner U, Kellner S, Weinitz S. Fundus autofluorescence (488 NM) and near-infrared autofluorescence (787 NM) visualize different retinal pigment epithelium alterations in patients with age-related macular degeneration. Retina. 2010;30(1):6-15. doi:10.1097/iae.0b013e3181b8348b
9. Midena E, Pilotto E. Microperimetry in age: related macular degeneration. Eye (Lond). 2017;31(7):985-994. doi:10.1038/eye.2017.34
10. Moschos MM, Nitoda E. The Role of mf-ERG in the Diagnosis and Treatment of Age-Related Macular Degeneration: Electrophysiological Features of AMD. Semin Ophthalmol. 2018;33(4):461-469. doi:10.1080/08820538.2017.1301496
11. Metrangolo C, Donati S, Mazzola M, et al. OCT Biomarkers in Neovascular Age-Related Macular Degeneration: A Narrative Review. J Ophthalmol. 2021;2021:9994098. Published 2021 Jul 17. doi:10.1155/2021/9994098
12. de Moraes CG, Song C, Liebmann JM, Simonson JL, Furlanetto RL, Ritch R. Defining 10-2 visual field progression criteria. Ophthalmology. 2014;121(3):741-749. doi:10.1016/j.ophtha.2013.10.018
13. Bjerager J, Schneider M, Potapenko I, et al. Diagnostic Accuracy of the Amsler Grid Test for Detecting Neovascular Age-Related Macular Degeneration : A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2023 ;141(4):315–323. doi:10.1001/jamaophthalmol.2022.6396
14. Hogg RE, Sivaprasad S, Wickens R, et al. Home-Monitoring Vision Tests to Detect Active Neovascular Age-Related Macular Degeneration. JAMA Ophthalmol. 2024;142(6):512–520. doi:10.1001/jamaophthalmol.2024.0918




