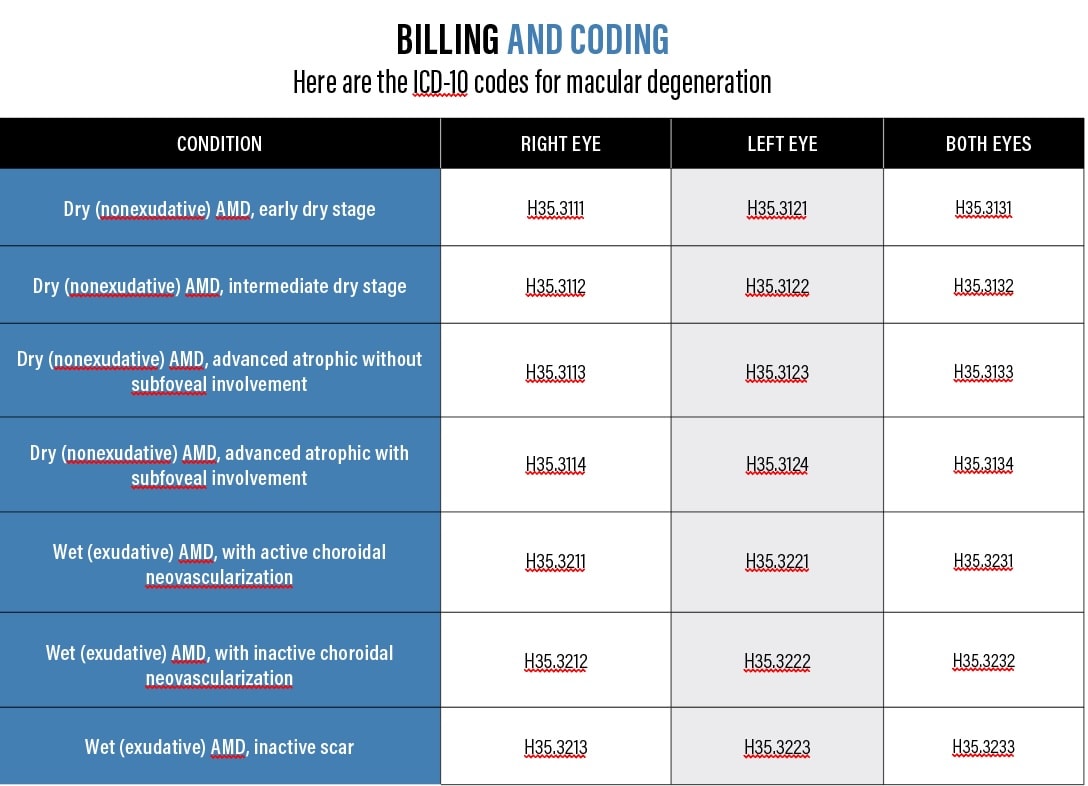
Geographic atrophy (GA) can lead to choroidal neovascularization (CNV), resulting in wet AMD. Additionally, a patient who has wet AMD may develop areas of GA. In either scenario, the patient will experience reduced vision, which can lead to progressive vision loss. Optometrists can identify the biomarkers that increase GA risk with the help of advanced diagnostic tools and inform these patients of interventions. (See "Billing and Coding" below.)
Here, I discuss these biomarkersas identified by the diagnostic tools available to me: color fundus photography (CFP), fundus autofluorescence (FAF), and optical coherence tomography (OCT). Additionally, I discuss the current interventions.
Geographic Atrophy Lesions
GA lesions are “sharply demarcated areas of retinal pigment epithelium (RPE) hypopigmentation, with clear visibility of underlying choroidal vessels.”1 On FAF, these lesions are hypofluorescent with surrounding hyperfluorescent lipofuscin accumulation1 (Figure 1). On OCT, these lesions show loss within the RPE, photoreceptors, and choriocapillaris layers, resulting in a “waterfall effect” appearance due to hypertransmission of light through the retina and into the choroid.
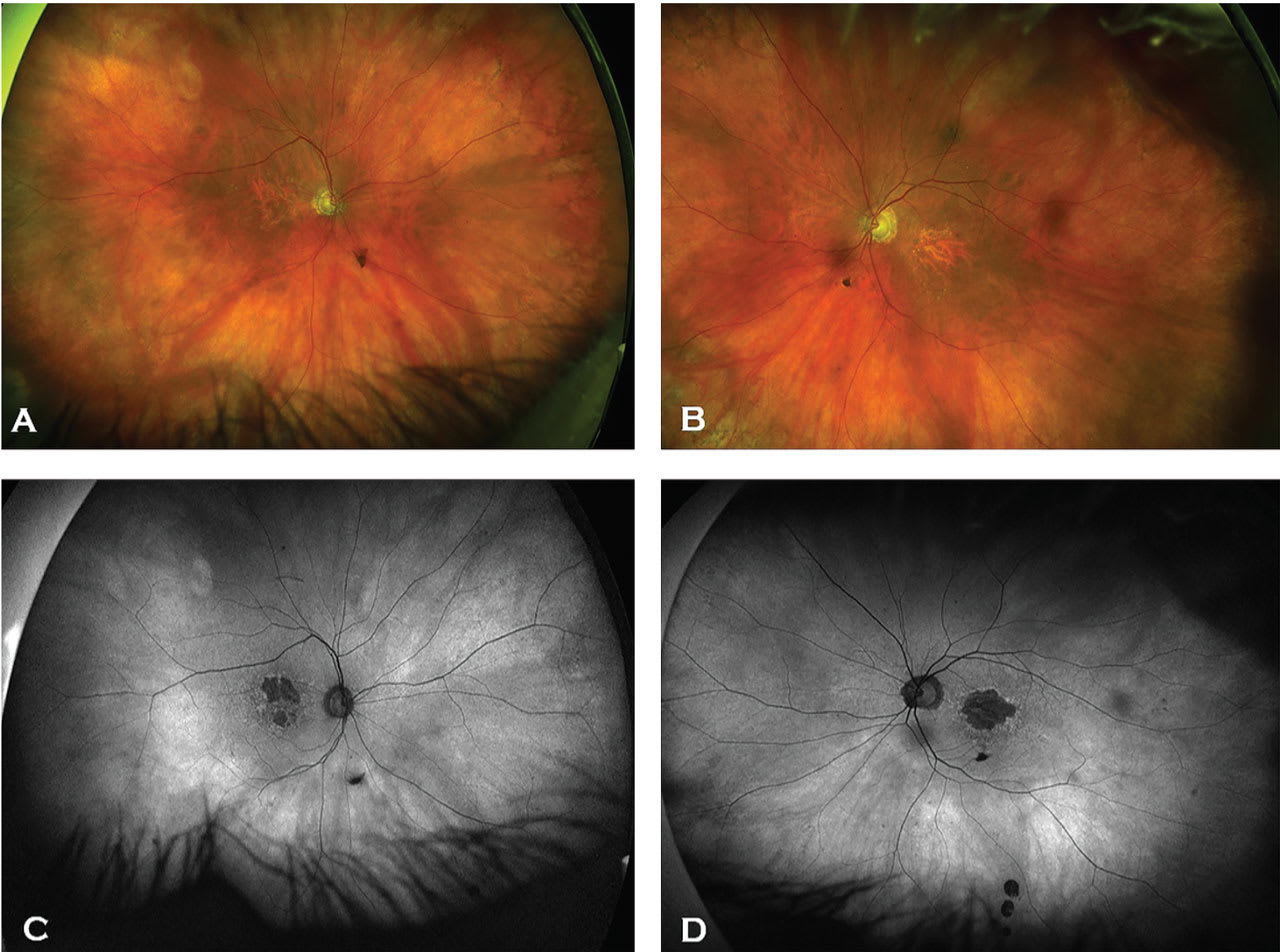
GA progression rates are higher with larger GA lesions than smaller ones, multifocal lesions vs unifocal lesions, extrafoveal lesions vs foveal lesions, larger hyperfluorescence around the lesion, and lesions that have more irregular margins at the junctional zone (irregular RPE elevations, RPE-Bruch’s membrane splitting, and thicker inner nuclear layer) than smooth margins.1
RPE and Outer Retinal Atrophies
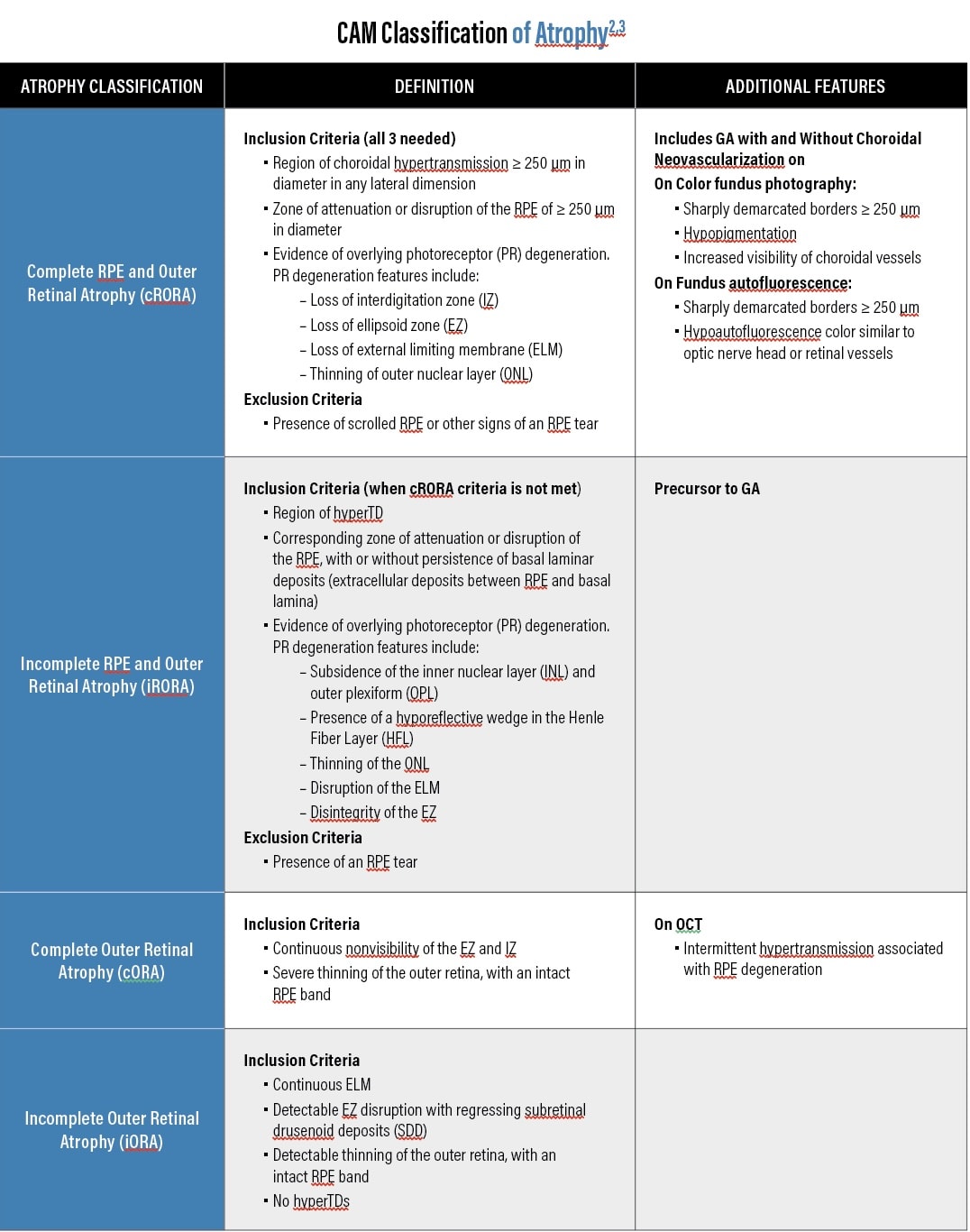
The Classification of Atrophy Meeting Group (CAM) developed definitions of atrophy (see CAM Classification of Atrophy," below) and assessed biomarkers in GA. Additionally, the group defined hypertransmission defects (hyperTDs) on OCT as “the presence of increased transmission of signal below the level of the RPE and into the choroid.”2
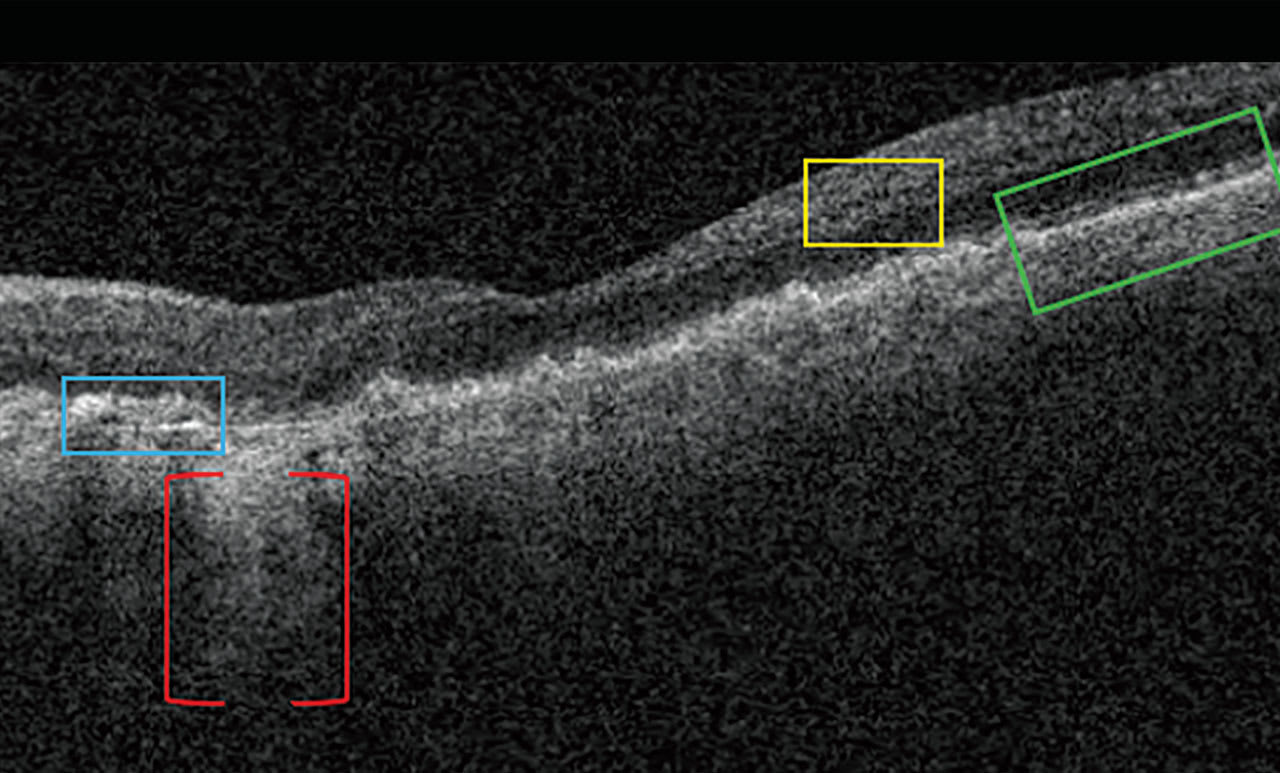
Nascent GA is drusen-associated incomplete RPE and outer retinal atrophy (iRORA) without CNV.1,2 On OCT, it appears as a subsidence of the outer plexiform layer (OPL) and the inner nuclear layer (INL), along with a wedge-shaped band within the OPL.1 The lesion’s boundaries are defined by “an abrupt increase in choroidal reflectivity below Bruch’s membrane; the RPE, photoreceptor, and choriocapillaris layer loss; and external limiting membrane absence/descent”.1 Also, nascent GA and iRORA increase progression risk to GA. In fact, eyes that have some of the findings of iRORA are at high risk of developing advanced AMD3 (Figure 2).
Drusen and Heterogeneous Internal Reflectivity Within Drusen
Patients who have drusen within 1500 μm of the fovea center at least 0.03 mm3 have a 4-fold increased risk of developing advanced AMD2 within 2 years.
On OCT, drusen appear as RPE elevations. Calcified drusen have a hyporeflective core surrounded by a hyperreflective area on OCT (Figure 3). Patients who have heterogeneous internal reflectivity within drusen (HIRD) have a 6-fold risk of progressing to advanced AMD within a year.4
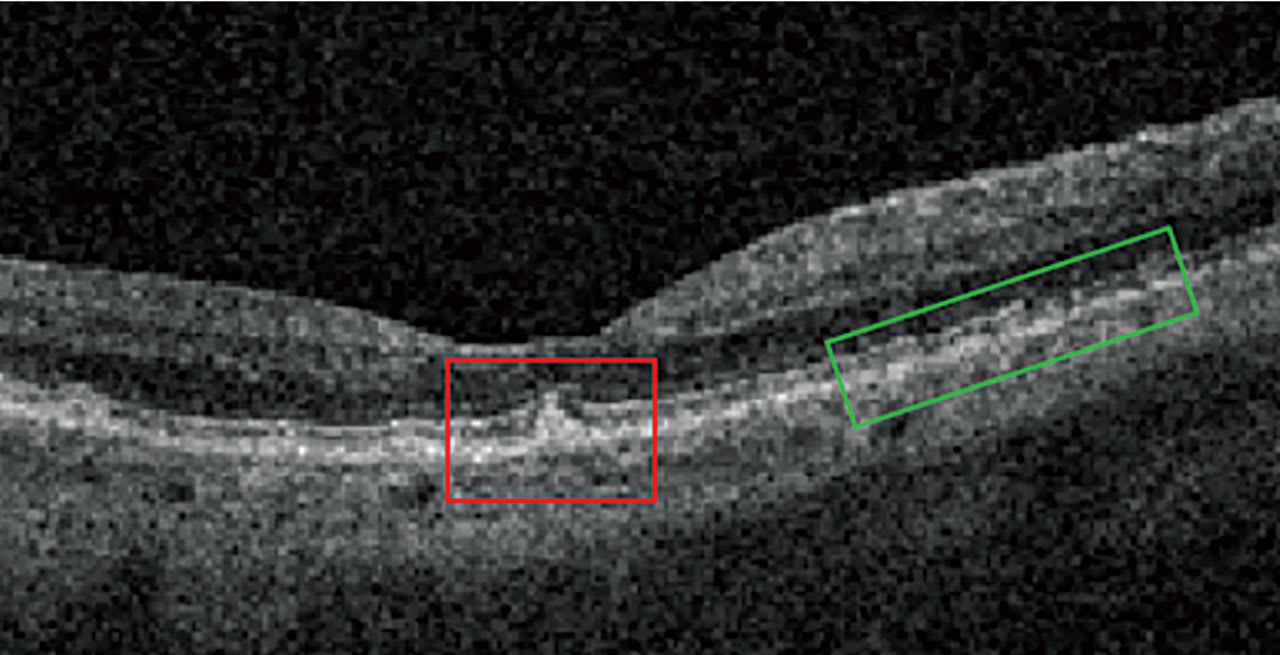
Reticular Pseudodrusen and Subretinal Drusenoid Deposits Reticular pseudodrusen (RPD), also called subretinal drusenoid deposits (SDD), are anterior to the RPE layer and have a reticular pattern of yellow-white lesions on CFP. RPD are associated with multifocal GA lesion development.1 On OCT (Figure 3), RPD are hyperreflective above the RPE (drusen are below the RPE). Patients who have RPD have a 2-fold increased risk of progressing to advanced AMD.5
Intraretinal Hyperreflective Foci (IHRF)
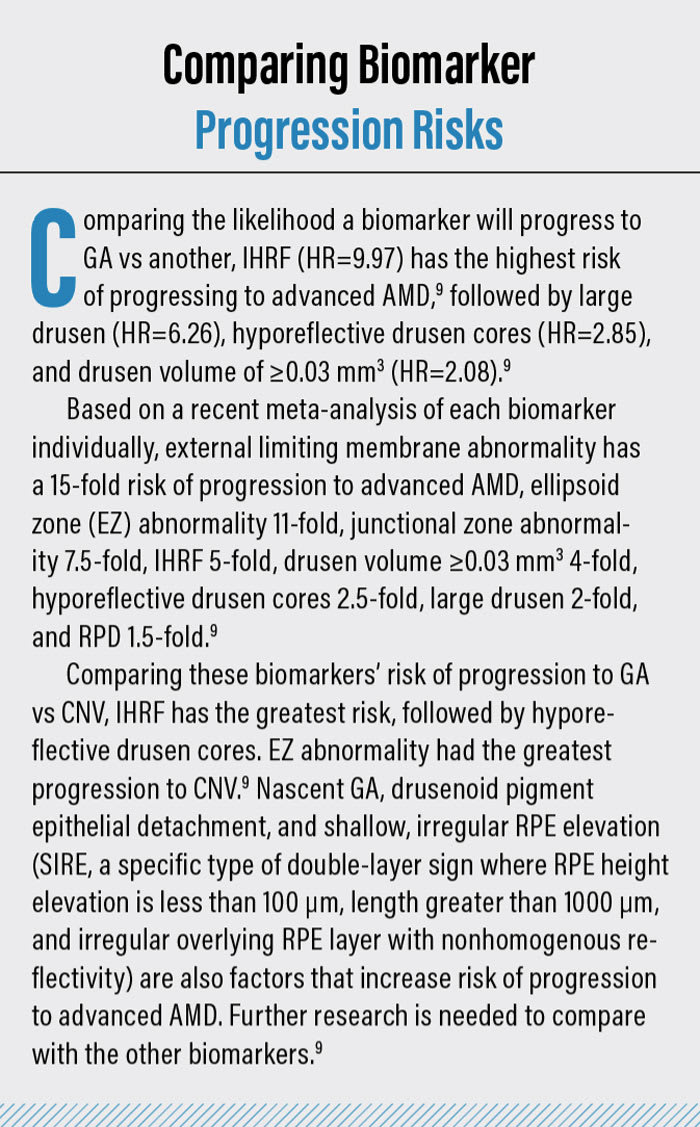
IHRF are small, punctate structures that are hyperreflective on OCT. They are mostly located in the INL and OPL. Patients with IHRF are at risk of developing GA within 2 years by 5-fold (see “Comparing Biomarker Progression Risks,” below).6
Current Interventions
• Home-based OCT. This allows for monitoring between follow-up visits, providing early detection of AMD progression.7
• Mediterranean-type diet.GA enlargement progression is slower in those who eat higher amounts of whole fruit, eat lower amounts of red meat, drink alcohol in moderation, and consume a higher ratio of monounsaturated fatty acids to saturated fatty acids (MUFA:SFA).8 Additionally, a Mediterranean diet reduces the risk of developing GA by 30%.8
• Ocular nutritional supplements. Supplements that contain vitamin C, vitamin E, zinc, copper, lutein, and zeaxanthin reduce the risk of intermediate AMD progression to advanced AMD by about 25%.9
• Smoking cessation. Current smokers are 4 times more likely to develop GA than nonsmokers.10 Past smokers have a 3 times higher risk.11 Smokeless tobacco users also have a greater risk.12 Further, exposure to second-hand smoke doubles a person’s risk of developing AMD.11
•Exercise. Regular exercise can reduce both the risk of early AMD and the progression to advanced AMD, specifically wet AMD.13 At least 3 hours of low to moderate physical activity per week can reduce the odds of late AMD by 41% compared to having a sedentary lifestyle.14
• Prescription medications. Avacincaptad pegol (Izervay, Astellas) and pegcetacoplan (Syfovre, Apellis Pharmaceuticals) treat GA. The former is a monthly intravitreal injection that slows GA progression by inhibiting the complement C5 protein, preventing other proteins from attacking retinal cells and photoreceptors, and, thus, reduces cell damage and vision loss. Adverse events include increased risk of wet AMD development, endophthalmitis, retinal detachment, retinal vasculitis, and increased intraocular pressure (IOP) after injection.15 The latter is a monthly or every-other-month intravitreal injection that inhibits complement factors 3 and 3b of the immune system’s complement cascade, thus reducing inflammation, vascular loss, and the cell damage that causes GA progression.16 Adverse events include endophthalmitis, retinal vasculitis, retinal detachments, an increased risk of wet AMD development, increased IOP after injection, intraocular inflammation, and ischemic optic neuropathy.17,18
• Photobiomodulation (PBM). This is a 9-session (over 3 to 5 weeks) noninvasive light therapy that decreases macular drusen volume, along with a 9.4 times less occurrence of new GA progression.18 The treatment incorporates specific wavelengths of light to target the retina at the cellular level by enhancing mitochondrial function to improve adenosine triphosphate production. Photobiomodulation is for patients “with a best-corrected visual acuity of 20/32 through 20/70 and who have dry age-related macular degeneration (AMD) characterized by: the presence of at least 3 medium drusen (>63 μm and ≤125 μm in diameter), or large drusen (>125 μm in diameter), or noncentral GA, and the absence of neovascular maculopathy or center-involving geographic atrophy.”18 PBM shows low adverse effects and no signs of phototoxicity.18
What’s Next?
There is research in the areas of cell replacement therapy19 and retinal implants20 to intervene on GA. Additionally, artificial intelligence could be helpful in identifying biomarkers. OM
References
1. Fleckenstein M, Mitchell P, Freund KB, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(3):369-390. doi:10.1016/j.ophtha.2017.08.038.
2. Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology. 2020;127(3):394-409. doi:10.1016/j.ophtha.2019.09.035
3. Trinh M, Cheung R, Duong A, Nivison-Smith L, Ly A. OCT Prognostic Biomarkers for Progression to Late Age-related Macular Degeneration: A Systematic Review and Meta-analysis. Ophthalmol Retina. 2024;8(6):553-565. doi:10.1016/j.oret.2023.12.006.
4. Agrón E, Domalpally A, Cukras CA, et al. Reticular Pseudodrusen: The Third Macular Risk Feature for Progression to Late Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology. 2022;129(10):1107-1119. doi:10.1016/j.ophtha.2022.05.021
5. Christenbury JG, Folgar FA, O’Connell RV, et al. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038-1045. doi:10.1016/j.ophtha.2012.10.014
6. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018;125(4):537-548.
7. Willis ET, Kim JE, Schneider EW. Home Optical Coherence Tomography Monitoring for Neovascular Age-Related Macular Degeneration: Transformative Technology or Cool Toy?. Ophthalmol Ther. 2024;13(6):1407-1416. doi:10.1007/s40123-024-00953-8
8. Agrón E, Mares J, Chew EY, Keenan TDL; AREDS2 Research Group. Adherence to a Mediterranean Diet and Geographic Atrophy Enlargement Rate: Age-Related Eye Disease Study 2 Report 29. Ophthalmol Retina. 2022;6(9):762-770. doi:10.1016/j.oret.2022.03.022
9. National Eye Institute. AREDS/AREDS2 Clinical Trials. https://www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-studies-aredsareds2/about-areds-and-areds2 (Accessed Sept. 12, 2025)
10. Tan JSL. Smoking and the Long-term Incidence of Age-Related Macular Degeneration. Archives of Ophthalmology. 2007;125(8):1089. doi:10.1001/ar chopht.125.8.1089
11. Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90(1):75-80. doi:10.1136/bjo.2005.073643
12. Ochoa A III, Amin SM, Loy KC, Yousuf SJ, Khan BA. Associations between electronic cigarettes, smokeless tobacco, and age-related macular degeneration in the 2017 National Health Interview Survey. Presented at: American Society of Retina Specialists Conference; July 28-Aug 1, 2023; Seattle, WA
13. McGuinness MB, Le J, Mitchell P, et al. Physical Activity and Age-related Macular Degeneration: A Systematic Literature Review and Meta-analysis. Am J Ophthalmol. 2017;180:29-38. doi:10.1016/j.ajo.2017.05.016
14. Danzig CJ, Khanani AM, Kaiser PK, et al. Vision Loss Reduction with Avacincaptad Pegol for Geographic Atrophy: A 12-Month Post Hoc Analysis of the GATHER1 and GATHER2 Trials. Ophthalmol Retina. 2024;8(11):1052-1060. doi:10.1016/j.oret.2024.04.023
15. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. The Lancet. 2023;402(10411):1434-1448. doi:10.1016/s0140-6736(23)01520-9
16. Charles C. Wykoff, Frank G. Holz, Allen Chiang, et al. Pegcetacoplan Treatment for Geographic Atrophy in Age-Related Macular Degeneration Over 36 Months: Data From OAKS, DERBY, and GALE. Am J Ophthalmol., 2025;276:350-364. doi.org/10.1016/j.ajo.2025.04.016
17. Boyer D, Hu A, Warrow D, et al. LIGHTSITE III: 13-Month Efficacy and Safety Evaluation of Multiwavelength Photobiomodulation in Nonexudative (Dry) Age-Related Macular Degeneration Using the Lumithera Valeda Light Delivery System. Retina. 2024;44(3):487-497. doi:10.1097/IAE.0000000000003980
18. Trinh M, Cheung R, Duong A, Nivison-Smith L, Ly A. OCT Prognostic Biomarkers for Progression to Late Age-related Macular Degeneration: A Systematic Review and Meta-analysis. Ophthalmol Retina. 2024;8(6):553-565. doi:10.1016/j.oret.2023.12.006
19. National Institutes of Health. First U.S. patient receives autologous stem cell therapy to treat dry AMD. https://www.nih.gov/news-events/news-releases/first-us-patient-receives-autologous-stem-cell-therapy-treat-dry-amd. Accessed Sept. 5, 2025.
20. Macular Degeneration Research. A BrightFocus Foundation Program. The Latest Developments in Retinal Implants.




