With the US Food and Drug Administration poised to complete its final review of epithelium (epi-on) corneal crosslinking (CXL), it makes sense to discuss what to expect from the procedure’s postoperative outcomes and how to approach contact lens fitting.
Postoperative Outcomes
The postoperative outcomes for epi-on CXL are stromal haze and cellular morphology changes. Regarding stromal haze, this usually occurs in the first month and resolves within 20 weeks. Additionally, a change of stromal refractive index will occur due to increased collagen fiber diameter and spacing. This is represented clinically as a stromal demarcation line that represents the depth of CXL tissue.
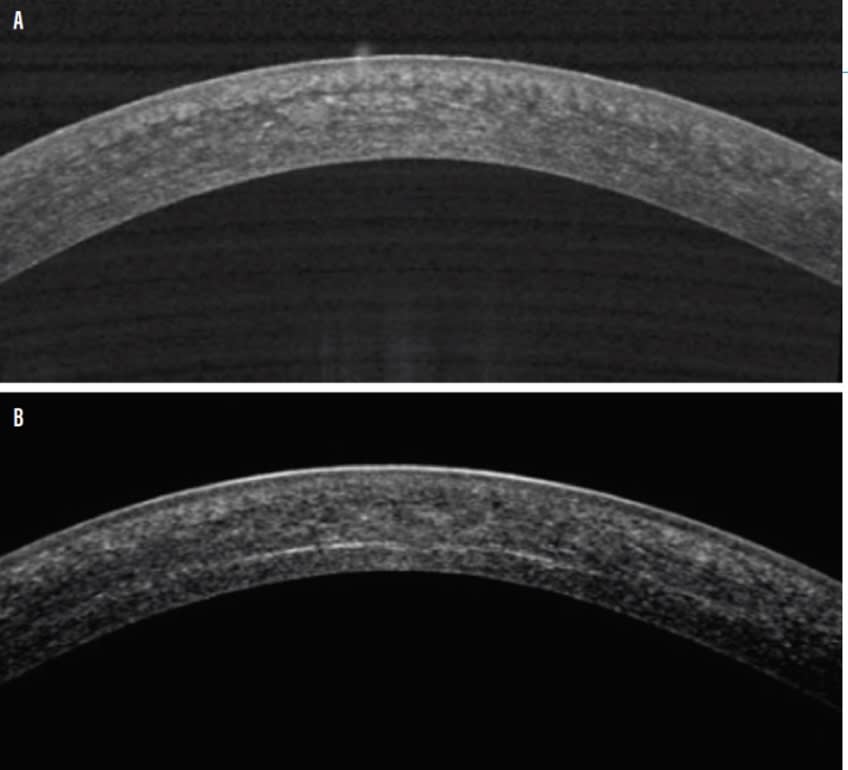
When it comes to cellular morphology changes, stromal edema and a reduction of sub-basal nerve fiber and keratocyte density are noted via in vivo confocal microscopy performed immediately after CXL. That said, these changes slowly resolve with corneal thickness and sub-basal nerve and stromal keratocytes density returning to baseline levels within 12 months.1
Contact Lens Fitting
I have had patients resume soft, rigid gas permeable, or hybrid contact lenses an average of 3 months postoperatively in my epi-off CXL patients. This is to allow for adequate epithelial healing.
Additionally, I have often fit or allowed patients to resume their scleral lenses as early as 3 weeks after their epi-off procedure if they can demonstrate the proper insertion and removal technique. This is because scleral lenses vault the fragile tissue.
For epi-on CXL patients, I will decrease these “wait times” to 1 month postoperatively for soft, rigid gas permeable, or hybrid contact lenses, and fit or enable patients to return to scleral lenses at the 2-week to 1-month postoperative visit.
Healing Rates
Of course, patients have individual healing rates and differing levels of urgency for visual rehabilitation. Therefore, care consideration of these factors will help guide us toward the safest and most successful clinical approach. OM
References
1. Glaukos. News Details. Glaukos Announces Positive Topline Outcomes in Phase 3 Confirmatory Trial for Epioxa™, Achieving Primary Efficacy Endpoint and Demonstrating Favorable Tolerability and Safety. Accessed July 24, 2025. https://investors.glaukos.com/investors/news/news-details/2024/Glaukos-Announces-Positive-Topline-Outcomes-in-Phase-3-Confirmatory-Trial-for-Epioxa-Achieving-Primary-Efficacy-Endpoint-and-Demonstrating-Favorable-Tolerability-and-Safety/default.aspx




